Introduction
Materials and Methods
1. Study design
2. Treatment plan
Table 1.
| JMII | S110 | PARAMOUNT | |
|---|---|---|---|
| Study design | Multicenter, open-label, single-arm post-marketing clinical trial | Phase II, multicenter, randomized, controlled, open-label study | Global, phase III, multicenter, randomized, double-blind, placebo-controlled study |
| Histology of patient population | |||
| Original analysis | NS NSCLC | SQ and NS NSCLC | NS NSCLC |
| Analysis for this review | NS NSCLC | NS NSCLC | NS NSCLC |
| Induction therapy | Pemetrexed 500 mg/m2 and carboplatin | Pemetrexed 500 mg/m2 and cisplatin 75 mg/2 every 21 days for 4 cycles | Pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 every 21 days for 4 cycles |
| AUC=6 every 21 days for 4 cycles | |||
| No. of patients enrolled/entered | 111 | 86a) | 1,022 |
| No. of patients treated with induction therapy | 109 | 70a) | 939 |
| No. of patients with NS disease | 109 | 59 | 939 |
| Randomization | No | Yes (prior to induction therapy) | Yes (prior to maintenance therapy) |
| Maintenance therapy | Pemetrexed 500 mg/m2every 21 days until PD | Optional cisplatin 75 mg/m2 for first 2 cycles and pemetrexed 500 mg/m2 every 21 days until PD/death/unacceptable toxicity | Pemetrexed 500 mg/m2 and BSC every 21 days until PD/unacceptable toxicity/decision of the patient or physician |
| No. of randomized patients to pemetrexed/treated with pemetrexed maintenance | 60 | 24b) | 359 |
| No. of patients with NS disease | 60 | 22 | 359 |
| Comparator | NA | Gefitinib 250 mg orally every day until PD/death/unacceptable toxicity | Placebo and BSC every 21 days until PD/unacceptable toxicity/decision of the patient or physician |
| No. of randomized patients to comparator group | NA | 25c) | 180 |
| Efficacy endpoints | |||
| Primary | PFS from enrollment date to PD or death (induction plus maintenance) | PFS from enrollment date to PD or death (induction plus maintenance); however, for the primary endpoint analysis, it was evaluated for the maintenance period only using a time-dependent Cox model | PFS from randomization date to PD or death (maintenance) |
| Secondary | OS, DCR, ORR, response rate in induction and DCR in maintenance | ORR, DoR, OS | OS, ORR |
| Baseline for efficacy and safety assessment | Enrollment | Randomization | Randomization |
| Efficacy analysis set | Treated patients with no significant protocol violation | Randomized and treated patients | Randomized patients |
| Safety analysis set | Patients who received at least one dose of pemetrexed and carboplatin induction therapy | Randomized and treated patients | Randomized patients |
| Adverse events assessment | AEs which onset newly or worsened in the entire treatment (induction+maintenance) in patients who received induction therapy | AEs which onset newly in maintenance or worsened from baseline and induction period | AEs which onset newly or worsened in the entire treatment (induction+maintenance) in patients who received the induction therapy |
| AEs which onset newly in maintenance or worsened from baseline and induction period | AEs which onset newly or worsened in the entire treatment (induction+maintenance) in patients who received the induction therapy | AEs which onset newly in maintenance or worsened from baseline and induction period | |
| PFS assessed in this manuscript | PFS for entire period-PFS for induction therapy period | PFS for maintenance period | PFS for only maintenance therapy |
NS, nonsquamous; NSCLC, non-small cell lung cancer; SQ, squamous; AUC, area under curve; PD, progressive disease; BSC, best supportive care; NA, not applicable; PFS, progression-free survival; OS, overall survival; DCR, disease control rate; ORR, objective response rate; DoR, duration of response; AE, adverse event; PC/G, pemetrexed+cisplatin followed by gefitinib; PC/P, pemetrexed+cisplatin followed by pemetrexed.
3. Eligibility criteria
Table 2.
| JMII | S110 | PARAMOUNT | |
|---|---|---|---|
| Age | ≥ 20 yr | ≥ 18 yr | ≥ 18 yr |
| ECOG PS | 0-1 | 0-1 | 0-1 |
| Stage | IIIB/IV, post-operative recurrent disease | IIIB/IV | IIIB/IV |
| Histology | NS NSCLC | SQ and NS NSCLC | NS NSCLC |
| Smoking status | NA | Never-smokera) | NA |
| EGFR mutation status | NA | Unknown | NA |
| CNS metastasis | Stable or treated brain metastasis allowed | Stable or treated brain metastasis allowed | Stable or treated brain metastasis allowed |
| Prior treatment history | Chemonaïve | Chemonaïve | Chemonaïve |
| Adjuvant excluded | Radiotherapy < 25% of the bone marrow | Adjuvant excluded | |
| Radiotherapy < 25% of the bone marrow | Radiotherapy < 25% of the bone marrow | ||
| Adequate organ function | Bone marrow reserve, renal, hepatic | Bone marrow reserve, renal, hepatic | Bone marrow reserve, renal, hepatic |
| Weight loss | NA | ≤ 10% within 6 weeks prior to study entry | NA |
| Measurable lesions | Measurable lesions meeting RECIST ver. 1.0 criteria | At least 1 unidimensionally measurable lesion meeting RECIST ver. 1.0 criteria | At least 1 unidimensionally measurable lesion meeting RECIST ver. 1.0 criteria |
| Criteria for randomization and/or receiving maintenance therapy | Evidence of CR, PR or SD after 4 cycles of induction therapy | Four cycles of induction therapy | Documented radiographic evidence of a tumor response of CR, PR, or SD after 4 cycles of induction therapy |
| Life expectancy | At least 12 weeks | Less than 12 weeks | At least 12 weeks |
ECOG PS, Eastern Cooperative Oncology Group performance status; NS, nonsquamous; NSCLC, non-small cell lung cancer; SQ, squamous; NA, not applicable/not available; EGFR, epidermal growth factor receptor; CNS, central nervous system; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease.
4. Efficacy and safety assessments
5. Statistical methods
Results
1. Baseline demographics and treatment
Table 3.
| Variable | JMII PCb/P (n=109) | S110 PC/P ind+mnt (n=27) | S110 PC/P mnt (n=22) | PARAMOUNT P+BSC mnt (n=359) |
|---|---|---|---|---|
| Age (yr) | ||||
| Median (range) | 63 (38-78) | 57.0 (29-75) | 54.5 (29-74) | 61 (32-79) |
| Gender | ||||
| Female | 40 (36.7) | 22 (81.5) | 17 (77.3) | 158 (44) |
| Male | 69 (63.3) | 5 (18.5) | 5 (22.7) | 201 (56) |
| Ethnic origin | ||||
| Asian | - | - | - | 16 (4) |
| Japan | 109 (100) | - | - | - |
| China | - | 13 (48.1) | 12 (54.5) | - |
| Korea | - | 6 (22.2) | 5 (22.7) | - |
| Taiwan | - | 8 (29.6) | 5 (22.7) | - |
| Caucasian | - | - | - | 339 (94) |
| African | - | - | - | 4 (1) |
| Stage of disease | ||||
| Stage IIIB | 33 (30.3) | 3(11.1) | 3 (13.6) | 31 (9) |
| Stage IV | 72 (66.1) | 24 (88.9) | 19 (86.4) | 328 (91) |
| Recurrence | 4 (3.7) | - | - | - |
| ECOG performance status | ||||
| 0 | 37 (33.9) | 8 (29.6) | 8 (36.4) | 115 (32) |
| 1 | 72 (66.1) | 19 (70.4) | 14 (63.6) | 243 (68) |
| 2-3 | - | - | - | 1 ( < 1) |
| Histological subtypes | ||||
| Adenocarcinoma | 106 (97.2)b) | 24 (88.9) | 21 (95.5) | 304 (85) |
| Large cell | 3 (2.8) | 1 (3.7) | - | 24 (7) |
| Bronchoalveolar | - | - | - | 6 (2) |
| Other | - | 2 (7.4) | 1 (4.5) | 25 (7) |
| Smoking | ||||
| Ever | 76 (69.7) | 2 (7.4) | 1 (4.5) | 275 (77) |
| Never | 33 (30.3) | 25 (92.6) | 21 (95.5) | 82 (23) |
| Unknown | 0 | 0 | - | 2 ( < 1) |
| EGFR mutation status | ||||
| Positive | 24 (22.0) | - | - | - |
| Negative | 63 (57.8) | - | - | - |
| Unknown | 3 (2.8) | - | - | - |
| Not done | 19 (17.4) | - | - | - |
Values are presented as number (%). PCb/P, pemetrexed+carboplatin followed by pemetrexed; PC/P, pemetrexed+cisplatin followed by pemetrexed; ind, induction; mnt, maintenance; P+BSC, pemetrexed+best supportive care; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer.
2. Dose administration of pemetrexed maintenance therapy
3. Efficacy
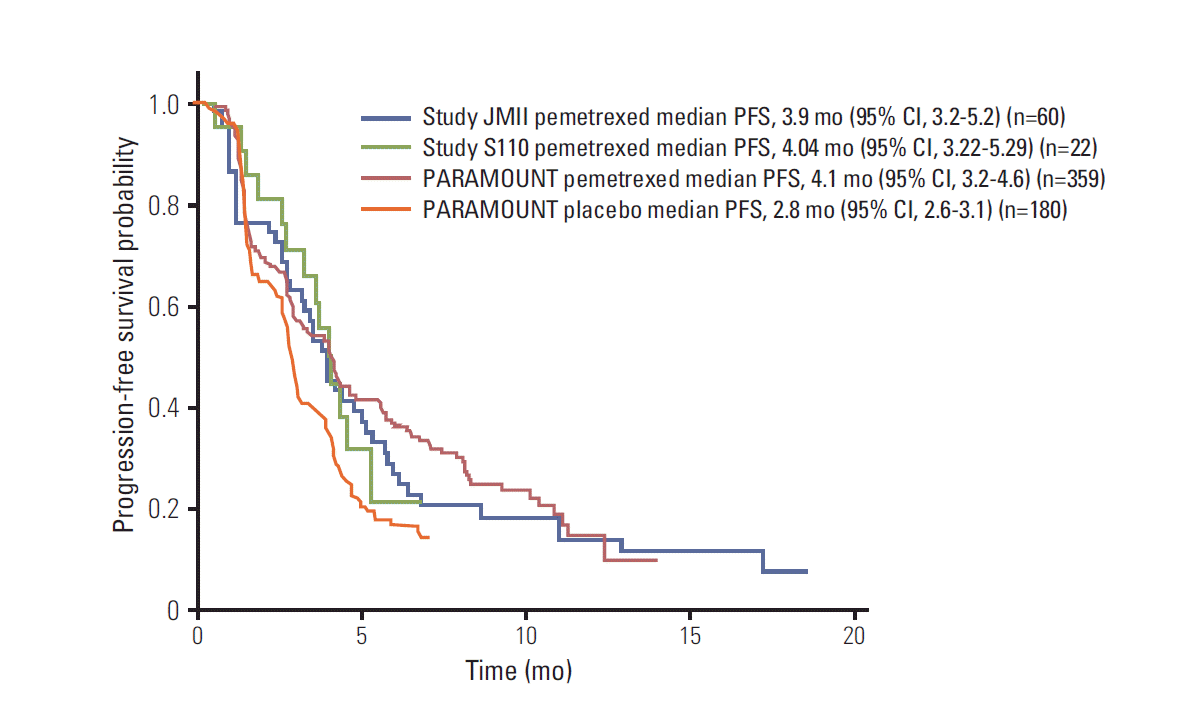 | Fig. 1.Kaplan-Meier curve of progression-free survival for maintenance therapy post-induction. These are 4 curves superimposed on one plot and, as this was not a randomized study of 4 arms, they should not be directly compared. CI, confidence interval; PFS, progression-free survival. |
Table 4.
| Efficacy parameter | JMII PCb/P | S110 PC/P | PARAMOUNT P+BSC mnt |
|---|---|---|---|
| Induction regimen | Carboplatin+pemetrexed | Cisplatin+pemetrexed | Cisplatin+pemetrexed |
| Induction sample size | 106 | 27 | 939 |
| Maintenance sample size | 60 (56.6%) | 22 (81.5%) | 359 (66.6%)a) |
| Efficacy during maintenance therapy | |||
| Maintenance regimen | Pemetrexed | Pemetrexed | Pemetrexed |
| No. | 60 | 22 | 359 |
| Median PFS (95% CI, mo) | 3.9 (3.2-5.2) | 4.04 (3.22-5.29) | 4.1 (3.2-4.6) |
| Efficacy during induction+maintenance period | |||
| No. | 106b) | 27 | 359a) |
| Median PFS (95% CI, mo) | 5.7 (4.4-7.3) | 6.83 (5.78-7.98) | 6.9 (6.2-7.5) |
| Median OS (95% CI, mo)c) | 20.2 (16.7-NA) | NRd) | 13.9 (12.8-16.0) |
| 1-Year survival rate (95% CI, %)c) | 70.0 (60.4-77.8) | 96.3 (76.5-99.5) | 58 (53.0-63.0) |
| 2-Year survival rate (95% CI, %)c) | 42.5 (32.8-51.8) | 78.0 (54.7-90.2) | 32 (27.0-37.0) |
| Subgroup analysis | |||
| Median PFS (95% CI, mo), EGFR mutation-positive patients | 5.7 (5.2-7.2) | NAe) | NAe) |
| Median PFS (95% CI, mo), EGFR mutation-negative patients | 6.9 (4.3-7.8) | NAe) | NAe) |
PCb/P, pemetrexed+carboplatin followed by pemetrexed; PC/P, pemetrexed+cisplatin followed by pemetrexed; P+BSC mnt, pemetrexed+best supportive care maintenance; PFS, progression-free survival; CI, confidence interval; OS, overall survival; NA, not available; NR, not reached; EGFR, epidermal growth factor receptor.
a) In PARAMOUNT, 939 patients were enrolled into the induction phase, and 539 patients (57.4%) (pemetrexed [n=359], placebo [n=180]) were randomized,
b) Efficacy assessment was performed on per-protocol set, which consisted of 106 treated patients without major protocol violations as reported in the JMII manuscript,
c) In PARAMOUNT, median OS, 1-year and 2-year survival rates are reported for the maintenance period but for the induction+maintenance period for S110 and JMII,
Table 5.
|
Entire treatment period |
Induction period |
|||
|---|---|---|---|---|
| JMII (n=106) | S110 (PC/P)(n=27) | JMII (n=106) | S110 (PC/P)(n=27) | |
| Best overall responsea) | ||||
| Complete response | 0 | 0 | 0 | 0 |
| Partial response | 38 (35.8) | 10 (37.0) | 42 (39.6) | 5 (18.5) |
| Stable disease | 41 (38.7) | 12 (44.4) | 45 (42.5) | 17 (63.0) |
| Progressive disease | 20 (18.9) | 3 (11.1) | 15 (14.2) | 2 (7.4) |
| Unknown | 7 (6.6) | 1 (3.7) | 4 (3.8) | 2 (7.4) |
| Early death from toxicity | NA | 1 (3.7) | 0 | 1 (3.7) |
| Disease control rate (CR+PR+SD) | 74.5 (65.1-82.5) | 81.5 (61.9-93.7) | 82.1 (73.4-88.8) | 81.5 (61.9-93.7) |
| Overall response rate (CR+PR) | 35.8 (26.8-45.7) | 37.0 (NA) | 39.6 (30.3-49.6) | 18.5 (6.3-38.1) |
4. Safety
Table 6.
| Grade ≥ 3 AE |
JMIIa) |
S110b) |
PARAMOUNTc),d),e) |
||
|---|---|---|---|---|---|
| PCb/P (n=109) | P maintenance (n=60) | PC/P (n=31) | P maintenance period (n=24) | P maintenance (n=359) | |
| Patients with ≥ 1 | - | - | - | - | 33 (9) |
| laboratory AE | |||||
| Hematologic AEs | - | - | 6 (19.4) | 3 (12.5) | - |
| Anemia | 34 (31.2) | 1 (1.7) | 1 (3.2) | 1 (4.2) | 16 (4) |
| Neutropenia | 62 (56.9) | 3 (5.0) | 5 (16.1) | 2 (8.3) | 13 (4) |
| Leukopenia | 24 (22.0) | 1 (1.7) | 1 | 1 | 6 (2) |
| Thrombocytopenia | 45 (41.3) | 0 | 0 | 0 | 4 (1) |
| Non-hematologic AEs | |||||
| Alanine aminotransferase | 7 (6.4) | 2 (3.3) | 1 (3.2) | 0 | 1 (<1) |
| Aspartate aminotransferase | 2 (1.8) | 1 (1.7) | 0 | 0 | 0 |
| Hypokalemia | 0 | 0 | 1 (3.2) | 0 | 0 |
| Hyponatremia | 0 | 0 | 1 (3.2) | 0 | 0 |
| Metabolic/laboratory, other | 0 | 0 | 2 (6.5) | 0 | 0 |
| Patients with ≥ 1 non-laboratory AE | - | - | - | - | 32 (9) |
| Fatigue (asthenia, lethargy, malaise) | 2 (1.8) | 2 (6.5) | 2 (6.5) | 15 (4) | |
| Nausea | 1 (0.9) | 3 (9.7) | 0 | 1 (< 1) | |
| Vomiting | 3 (2.8) | 4 (12.9) | 0 | 0 | |
| Mucositis or stomatitis | 0 | 0 | 0 | 1 (< 1) | |
| Anorexia/appetite loss | 6 (5.5) | 1 (3.2) | 0 | 1 (< 1) | |
| Pain, any event | 0 | 0 | 0 | 3 (< 1) | |
| Infection | 0 | 0 | 0 | 4 (1) | |
| Neuropathy: sensory | 0 | 0 | 0 | 1 (< 1) | |
| Dyspnea | 0 | 2 (6.5) | 1 (4.2) | 0 | |
| Rash | 1 (0.9) | 0 | 0 | 0 | |
| Pneumonitis | 0 | 1 (3.2) | 0 | 0 | |
| Fever | 1 (0.9) | 0 | 0 | 0 | |
Values are presented as number (%). PCb/P, pemetrexed+carboplatin followed by pemetrexed; PC/P, pemetrexed+cisplatin followed by pemetrexed (10 patients also received optional cisplatin); AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events; NSCLC, non-small cell lung cancer; P, pemetrexed; TEAEs, treatment-emergent adverse events.
a) Toxicities in study JMII were TEAEs based on MedDRA ver. 14.1 and NCI-CTCAE ver. 3.0 preferred terms,
b) Toxicities in study S110 were based on NCI-CTCAE ver. 3.0; 4 patients had squamous NSCLC on PC/P induction+P maintenance and 2 patients had squamous NSCLC on PC/P maintenance period. There has been no evidence to date that toxicity varies by histology,
d) Among possibly drug-related AEs during the maintenance treatment period, no laboratory AEs of grade 5 (deaths) and 2 non-laboratory AEs of grade 5 (deaths) were recorded: 1 patient died in the pemetrexed group (pneumonia) and 1 patient died in the placebo group (sudden death—not otherwise specified),
5. Post-discontinuation anti-cancer therapy
Table 7.
| Post-study anti-cancer therapya) |
JMII |
S110b) |
PARAMOUNTc) |
|
|---|---|---|---|---|
| All treated patients (n=109) | Maintenance treated patients (n=60) | PC/P entire period (n=31) | Pemetrexed (n=359) | |
| Any post-study anti-cancer therapy | 86 (78.9) | 50 (83.3) | 25 (80.6) | 231 (64) |
| Surgery | 0 | 0 | 1 (3.2) | - |
| Radiotherapy | 9 (8.3) | 3 (2.8) | 13 (41.9) | - |
| Chemotherapy (≥ 1 line) | - | - | 18 (58.1) | - |
| Pemetrexed | 11 (10.1) | 4 (6.7) | 0 | 7 (2) |
| Docetaxel | 43 (39.4) | 24 (40.0) | 10 (32.3) | 116 (32) |
| Other | 59 (54.1) | 32 (53.3) | 11 (35.5) | 96 (27) |
| Biological (targeted therapy) | 34 (31.2) | 19 (31.7) | 18 (58.1) | - |
| Erlotinib | 19 (17.4) | 11 (18.3) | 5 (16.1) | 142 (40) |
| Gefitinib | 16 (14.7) | 8 (13.3) | 14 (45.2) | 3 (0.8) |
| Vandetanib | 0 | 0 | 3 (9.7) | 0 |
| BIBW2992 | 0 | 0 | 4 (12.9) | 0 |
| Sorafenib | 0 | 0 | 0 | 0 |
| Cetuximab | 0 | 0 | 1 (3.2) | 0 |
| Bevacizumab | 8 (7.3) | 4 (6.7) | 1 (3.2) | 6 (2) |
| Afatinib | 0 | 0 | 0 | 2 (0.6) |
| Immunological | 0 | 0 | 1 (3.2) | 0 |
| Investigational drug | 9 (8.3) | 8 (13.3) | 0 | 20 (6) |
| Placebo | 0 | 0 | 0 | 4 (1) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download