This article has been
cited by other articles in ScienceCentral.
Abstract
Here we report a case of a 76-year-old man with diffuse large B-cell lymphoma (DLBCL) with simultaneous involvement of the right breast and left testicle. The patient underwent complete resection of the involved testis, followed by immunochemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) and prophylactic radiotherapy to the contralateral testis. Following this multimodal therapy, he achieved a complete response. This is a rare case of DLBCL involving both the breast and the testis in a male patient.
Go to :

Keywords: Diffuse large B-cell lymphoma, Breast, Testis, Male
Introduction
Primary extranodal non-Hodgkin’s lymphoma (NHL) is uncommon. Extranodal NHL can occur in any visceral or non-visceral organ, but there are several predominant sites, including the stomach, small intestine, skin, and brain. NHL in the breast or testis is relatively rare, representing 0.38%-0.7% and 1%-2% of all NHL, respectively [
1,
2]. These types of lymphoma are generally high-grade lymphomas with an aggressive clinical course. The main treatment strategy for diffuse large B-cell lymphoma (DLBCL) with involvement of the breast is an anthracycline-containing chemotherapy with or without radiotherapy. The role of central nervous system (CNS) prophylaxis remains controversial despite the risk of CNS relapse, due to unproven benefit. On the other hand, anthracycline-based chemotherapy combined with locoregional radiotherapy to the contralateral testis and CNS prophylaxis is recommended in testicular DLBCL [
2]. The 5-year overall survival rate from previous reports varies from 26% to 71% in breast lymphoma and 56%-87% in testicular lymphoma. Here we report a patient with DLBCL involving both the breast and testis who was successfully treated with complete resection of the involved testis; immunochemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP); and prophylactic radiotherapy to the contralateral testis.
Go to :

Case Report
A 76-year-old man visited our hospital (Asan Medical Center, Seoul, Korea) with a mass on his right breast first being recognized two months ago. He had no B symptoms and an unremarkable medical history, except for hypertension. On physical examination he had a rubbery, non-tender, mobile mass of approximately 4 cm on his right breast. The mass was approximately 1 cm when it was first detected by the patient. The skin over the right areolar and subareolar portion was indurated with redness. There were no other palpable lymph nodes in the axillary, neck, or inguinal area, but his right testis was slightly harder than the left testis, without a definite mass.
Routine complete blood count and chemical laboratory data were all within normal range. The level of lactate dehydrogenase was 191 IU/L, and β2 microglobulin was 1.8 μg/mL. Screening tests for human immunodeficiency virus, hepatitis C virus, and hepatitis B virus were all negative.
On mammography, a heterogeneous, dense, lobulated mass-like lesion was observed in the subareolar portion of the right breast. The pathologic specimen from core needle biopsy revealed diffuse proliferation of large, atypical lymphoid cells (
Fig. 1A). Subsequent immunohistochemical staining was positive for CD20 (
Fig. 1B), Bcl-6, and IRF4/MUM1, but was negative for CD10.
In situ hybridization for Epstein-Barr virus was also negative. Based on these pathologic findings, the patient was diagnosed with DLBCL.
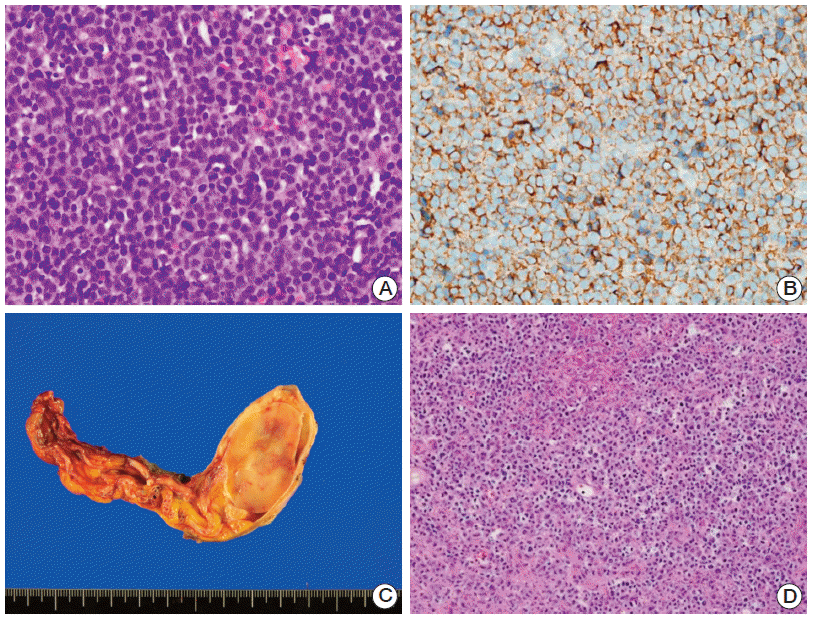 | Fig. 1.(A) Hematoxylin and eosin (H&E)–stained image showing diffuse large B-cell lymphoma of the right breast. (B) Immunohistochemical staining with positivity for CD20. (C) Gross specimen of the right testis, epididymis, and spermatic cord with an ovoid mass (3.8×2.5×1.6 cm). (D) H&E-stained image consistent with diffuse large B-cell lymphoma of the testis. 
|
A staging work-up including bone marrow biopsy with aspirate, positron emission tomography–computed tomography (PET-CT), and abdomen and chest computed tomography (CT) revealed no evidence of other nodal or marrow involvement. However, the PET-CT showed increased metabolism in the right testis, with a maximum standardized uptake value of 8.4 (
Fig. 2). He underwent radical orchiectomy as a diagnostic and therapeutic option. Pathologic examination revealed a well-defined ovoid mass (3.8×2.5×1.6 cm), which was confirmed as DLBCL (
Fig. 1C and
D). Based on these findings, he was finally diagnosed with DLBCL involving both the breast and testis, with an Ann Arbor clinical stage of 4EA. His international prognostic index was 3.
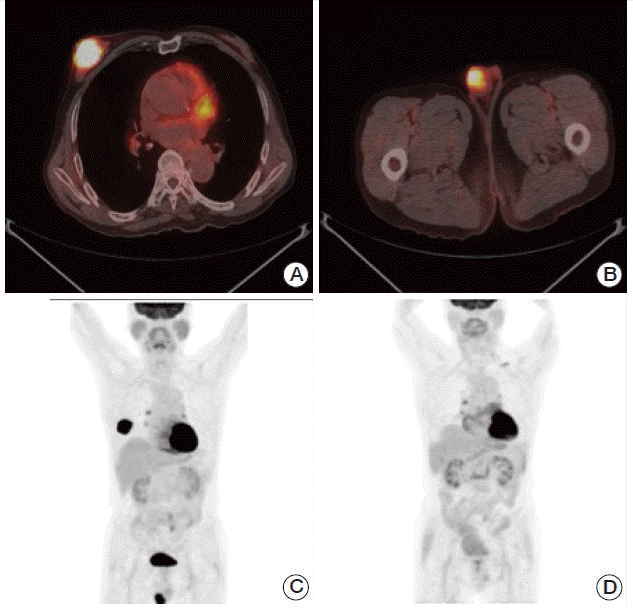 | Fig. 2.A-C) Positron emission tomography–computed tomography (PET-CT) showing hypermetabolism in the right breast and right testis. (D) PET-CT after four cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP), showing a complete response. 
|
The patient received a total of six cycles of immunochemotherapy (375 mg/m2 rituximab on day 1, 750 mg/m2 cyclophosphamide on day 1, 50 mg/m2 doxorubicin on day 1, 1.4 mg/m2 vincristine on day 1, and 50 mg prednisolone bid on days 1-5 [R-CHOP]). Follow-up CT and PET-CT after four cycles and six cycles of R-CHOP immunochemotherapy confirmed a complete response. After six cycles of R-CHOP immunochemotherapy, prophylactic radiotherapy of 25 Gy with 10 fractions was administered to his left testis. He has been followed at the outpatient clinic regularly without evidence of relapse for 17 months.
Go to :

Discussion
Extranodal NHL of the breast is uncommon, and its occurrence in male patients is extremely rare [
1].
Table 1 provides an overview of previously reported cases of male primary breast lymphoma (PBL) [
3-
13]. None of these cases presented with metastatic disease at first presentation, but three patients relapsed at other sites, including the CNS, bone marrow, cervical lymph nodes, and adrenal glands. The majority [
3,
4,
8,
10,
11,
13] of patients were diagnosed with DLBCL.
Table 1.
Previous reported cases of male primary breast lymphoma, including the present case
|
Author |
Age (yr) |
Location/Size (cm) |
Stage |
Histology |
Treatment |
Site of relapse |
Prognosis |
|
Docimo et al. (1959), cited by Sashiyama et al. [3] |
9 |
- |
- |
- |
- |
- |
Local recurrence |
|
Sinner et al. (1961), cited by Sashiyama et al. [3] |
35 |
R/Walnut |
- |
- |
RM+RT |
- |
9 yr alive |
|
Bettini et al. (1964), cited by Sashiyama et al. [3] |
38 |
R/Tangerine |
- |
- |
LE+RT |
- |
- |
|
Aoi et al. (1985), cited by Sashiyama et al. [3] |
81 |
R/4×4 |
IE |
DSNC |
SM |
- |
- |
|
Yamamoto et al. (1986), cited by Sashiyama et al. [3] |
65 |
L/4×5×2 |
IE |
DLBCL |
SM+CT |
- |
1 yr 8 mo alive |
|
Kawanishi et al. (1989), cited by Sashiyama et al. [3] |
74 |
R/>2.0 |
IE |
DLBCL |
RM+CT |
- |
- |
|
Hugh et al. (1990), cited by Sashiyama et al. [3] |
81 |
B |
IE |
DLBCL |
CT |
- |
5 mo died |
|
Murakami et al. (1993), cited by Sashiyama et al. [3] |
45 |
B/5.6×5×3.5, 4×3.5×2.2 |
IISE |
DLBCL |
LE |
- |
5 mo alive |
|
Kitaoka et al. (1993), cited by Sashiyama et al. [3] |
65 |
L/7×5×5 |
IE |
DLBCL |
RM+CT+RT |
- |
5 mo alive |
|
Okada et al. (1995), cited by Sashiyama et al. [3] |
69 |
R/11×11×8 |
- |
DM |
RM+CT+RT |
- |
1 yr 6 mo died |
|
Murata et al. (1996) [4] |
76 |
R/7×6×4 |
IE |
DLBCL |
RM+CT |
- |
39 mo alive |
|
Kohara et al. (1997) [5] |
45 |
B |
IVE |
DLBCL |
CT (CHOP) |
CNS, BM |
4 yr died |
|
Sashiyama et al. (1999) [3] |
69 |
L/2.4×3.9 |
IE |
DLBCL |
RM+CT |
- |
1 yr alive |
|
Kim et al. (1999) [6] |
64 |
R/3×4 |
IIEA |
- |
Lu+RT |
Neck, axilla |
4 yr died |
|
Cabras et al. (2004) [7] |
44 |
L/2 |
IIEA |
DLBCL |
LE+RT+CT (ACOP-B) |
- |
123 mo alive |
|
Mpallas et al. (2004) [8] |
67 |
R/6×5×4 |
IE |
DLBCL |
CT |
Adrenal |
1 yr died |
|
Gualco et al. (2009) [9] |
65 |
R/3 |
- |
ALCL |
- |
- |
- |
|
Ryan et al. (2008) [10] |
- |
- |
- |
FL |
- |
- |
- |
|
Duman et al. (2011) [11] |
62 |
L/7×4 |
IIE |
MZL |
CT (R-CHOP) |
- |
- |
|
Alhabshi et al. (2011) [12] |
26 |
R/6×5×4 |
IE |
B-cell type |
CT (CHOP)+RT |
- |
2 yr alive |
|
Rathod et al. (2011) [13] |
48 |
L/3.8×3.5×2.8 |
- |
DLBCL |
CT (CHOP) |
- |
- |
|
Present case |
77 |
R/5.8×3.5 |
IVEA (testis) |
DLBCL |
CT (R-CHOP)+RT to testis |
- |
- |

The prognosis of PBL is somewhat variable, with the 5-year survival rate ranging from 26%-71% [
1]. According to some reports, progression to CNS is relatively more common in PBL than in other NHL, ranging from 12%-27% [
1,
14]. Recent studies, however, suggest that routine CNS prophylaxis should not be given despite the high risk of CNS relapse in PBL, because there is no survival benefit [
1,
10].
In primary testicular lymphoma (PTL), previous studies report a continuous pattern of relapse, even at 10-14 years after initial therapy. Similar to PBL, common sites of relapse include extranodal sites such as the CNS and contralateral testis. The risk of CNS relapse is reported to be 20% and 35% at 5 and 10 years, respectively. Although bilateral involvement is uncommon at diagnosis, up to 35% of PTL patients develop contralateral testicular involvement later in their clinical course, which provides a rationale for prophylactic radiotherapy to the contralateral testis.
Anthracycline-containing chemotherapy (i.e., CHOP) is considered the standard treatment option for early-stage DLBCL. A number of cases benefitted from radiotherapy upon relapse on the contralateral side, which improved overall and progression-free survival in PTL [
2]. The role of additional radiotherapy to the involved side of breast, however, is yet to be determined, although it did show positive effects on progression-free survival and the risk of ipsilateral locoregional progression [
15].
Here, we present the first reported case of DLBCL with simultaneous breast and testicular involvement, without any nodal presentation. The patient received immunochemotherapy (R-CHOP) and prophylactic radiotherapy to the contralateral testis because of the high relapse rate of testicular lymphoma. In summary, DLBCL involving both the breast and testis was successfully treated with multimodal strategies and showed a favorable response without evidence of recurrence.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download