Abstract
Purpose
The response to haloperidol as a first-line neuroleptic and the pattern of neuroleptic rotation after haloperidol failure have not been well defined in palliative care. The purpose of this study was to determine the efficacy of haloperidol as a first-line neuroleptic and the predictors associated with the need to rotate to a second neuroleptic.
Materials and Methods
We conducted a retrospective review of the charts of advanced cancer patients admitted to our acute palliative care unit between January 2012 and March 2013. Inclusion criteria were a diagnosis of delirium and first-line treatment with haloperidol.
Results
Among 167 patients with delirium, 128 (77%) received only haloperidol and 39 (23%) received a second neuroleptic. Ninety-one patients (71%) who received haloperidol alone improved and were discharged alive. The median initial haloperidol dose was 5 mg (interquartile ranges [IQR], 3 to 7 mg) and the median duration was 5 days (IQR, 3 to 7 days). The median final haloperidol dose was 6 mg (IQR, 5 to 7 mg). A lack of treatment efficacy was the most common reason for neuroleptic rotation (87%). Significant factors associated with neuroleptic rotation were inpatient mortality (59% vs. 29%, p=0.001), and being Caucasian (87% vs. 62%, p=0.014). Chlorpromazine was administered to 37 patients (95%) who were not treated successfully by haloperidol. The median initial chlorpromazine dose was 150 mg (IQR, 100 to 150 mg) and the median duration was 3 days (IQR, 2 to 6 days). Thirteen patients (33%) showed reduced symptoms after the second neuroleptic.
Delirium, which is one of the most distressing syndromes in terminal cancer patients [1-3], is characterized by acute confusion, an altered level of consciousness, restlessness, decreased cognition, and abnormal perception, all of which tend to fluctuate over the course of the day [1,4]. Delirium is associated with a higher rate of morbidity and mortality, longer hospital stay, higher health care costs, and significant distress to patients, family members, and professional caregivers [5,6]. The current management of delirium involves identifying and removing any potentially reversible causes and prescribing pharmacologic and non-pharmacologic interventions [7]. Non-pharmacologic measures such as environmental controls and aids for orientation are recommended [5,8,9]. However, if these fail to alleviate symptoms, treatment with pharmacologic neuroleptics (e.g., haloperidol, chlorpromazine, olanzapine, aripiprazole, and quetiapine) and/or benzodiazepines are recommended [5,8,10-13]. The optimal order and dose of neuroleptics for delirium has not been well defined [14]. In our acute palliative care unit (APCU), haloperidol is mainly used as a firstline treatment, followed by rotation to chlorpromazine if patients continue to experience agitated delirium; however, the rate of response to haloperidol as a first-line neuroleptic has not been well defined in a palliative care setting. We recently documented the lack of efficacy of low dose haloperidol in alleviating delirium recall and related distress [15,16]. A better understanding of the effectiveness of firstline haloperidol will help optimize the management of delirium in palliative care. In this study, we evaluated the efficacy of haloperidol as a first-line neuroleptic in cancer patients with delirium and the predictive factors associated with the need for second-line therapy.
This study was approved by the Institutional Review Board at Texas University of Texas MD Anderson Cancer Center with a waiver for informed consent. We conducted a retrospective review of the charts of 167 consecutive patients with advanced cancer who were admitted to our APCU between January 1, 2012, and March 31, 2013. Inclusion criteria were (1) a diagnosis of delirium based on clinical diagnosis by a palliative medicine specialist or a score ≥ 7/30 on the Memorial Delirium Assessment Scale (MDAS) and (2) treatment with haloperidol as a first-line neuroleptic for delirium.
We collected the following information from electronic medical records: demographics (age, sex, race, and performance status), cancer diagnosis, duration of APCU stay, source of admission (oncology service through the consultation team, outpatient ambulatory center, or the emergency center), discharge information (discharged alive or died in the APCU), delirium characteristics (MDAS score and subtype), neuroleptic treatment information, concurrent benzodiazepine treatment status, Edmonton Symptom Assessment Scale (ESAS) score upon admission to the APCU, CAGE (i.e., cut down, annoying, guilty, and eye-opener), do-not-resuscitate status at admission, response to neuroleptics, and the haloperidol equivalent daily dose (HEDD) [16]. To facilitate comparisons of patients who received multiple neuroleptics, we calculated the HEDD using the defined daily dose [17], which is a theoretical unit of measurement defined as the assumed standard daily dose for a drug used for its main indication in adults. The defined daily doses for haloperidol (oral or parenteral), chlorpromazine (parenteral), and olanzapine (oral) are 8 mg, 100 mg, and 10 mg, respectively. The ESAS score is a widely used and validated tool for assessment of nine symptoms (pain, fatigue, nausea, depression, anxiety, drowsiness, appetite, shortness of breath, and sleep) and general feeling of well-being on a scale of 0 (no symptoms) to 10 (worst symptoms imaginable) [18].
Our primary objective was to determine the proportion of patients with delirium for whom haloperidol failed, which was defined as the number of patients requiring second-line neuroleptics. To document the use of haloperidol in clinical practice, standard descriptive statistics including means, median, interquartile ranges (IQR), standard deviations, and ranges, were used together with 95% confidence intervals. We also analyzed the daily neuroleptics dose using HEDD. We determined the degree of correlation between mean HEDD and continuous clinical parameters using Spearman’s rank correlation coefficients. The statistical significance of differences of various variables between patients who required neuroleptic rotation and those who did not were calculated using the Kruskal-Wallis, chi-square, and Fisher exact tests. Multivariate analysis was performed using logistic regression with backward elimination. SAS ver. 9.2 (SAS Institute, Cary, NC) and R ver. 2.3.1 (The R Foundation for Statistical Computing, Vienna, Austria) were used to perform these analyses.
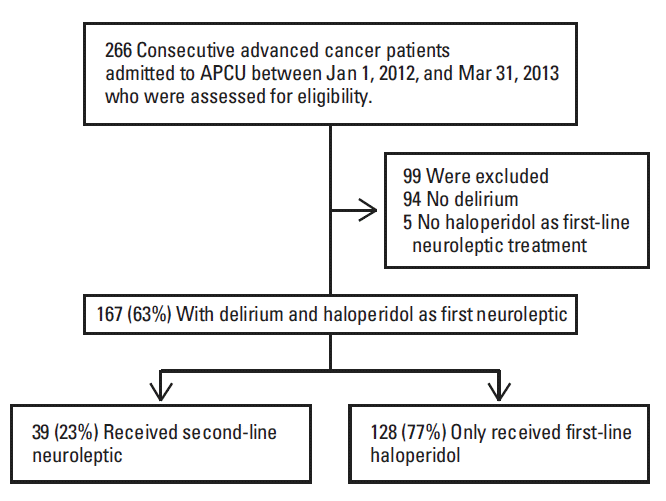
Of the 266 patients assessed for eligibility, 167 were included for analysis (Fig. 1). The patient demographics are summarized in Table 1. Ninety-two of the patients (55%) were transferred from Department of Oncology, while 58 (35%) were admitted from the emergency center. Among the 167 patients, the inpatient mortality rate was 36%. Among patients discharged alive, 52 (49%) were sent home or to home hospice, 49 (46%) to inpatient hospice, and 6 (6%) to another hospital. All patients had stage 4 cancer.
Of the 167 patients with delirium, 128 (77%) received only haloperidol and 39 (23%) needed a second neuroleptic. Among these 39 patients, 33 (85%) stopped haloperidol and started another neuroleptic, and six (15%) added a second neuroleptic to the existing haloperidol regimen. Ninety-one patients (71%) who received haloperidol alone showed symptom improvement and were discharged alive. The initial median daily haloperidol dose was 5 mg (IQR, 3 to 7 mg) and the median duration was 5 days (IQR, 3 to 7 days). The final median haloperidol dose was 6 mg (IQR, 5 to 7 mg). Lack of treatment efficacy was the most common reason for neuroleptic rotation (87%). As shown in Table 2, significant factors associated with neuroleptic rotation included inpatient mortality (59% vs. 29%, p=0.001), and white race (87% vs. 62%, p=0.014). Cancer diagnosis, stage of cancer, CAGE, duration of APCU stay, delirium subtype, do-not-resuscitate status at admission, admission source, and symptom burdens were not associated with neuroleptic rotation. Benzodiazepines were administered concurrently to most patients (70%). Among benzodiazepines, lorazepam was most commonly used (93%). The duration of first neuroleptic treatment, the haloperidol dose, and concurrent benzodiazepine administration were not associated with the need for second-line neuroleptic therapy.
Treatment efficacy was the most common reason for second-line neuroleptic (87%), whereas adverse events accounted for 13% of the cases. Chlorpromazine was administered to 37 patients (95%) who required second-line neuroleptic. The initial median daily chlorpromazine dose was 150 mg (IQR, 100 to 150 mg) and the median duration of second-line neuroleptic treatment was 3 days (IQR, 2 to 6 days). Only 13 patients (33%) improved after the second neuroleptic treatment. The Spearman’s rank correlation between the final dose of haloperidol and various clinical measures, revealed that the MDAS at admission (correlation coefficient, 0.24; p=0.005) and duration of first-line neuroleptic treatment (correlation coefficient, –0.19, p=0.01) were significantly correlated with the final dose of haloperidol. Finally, we attempted to identify independent predictors of rotation to second-line neuroleptic treatment. We included all variables that were identified as significantly different (or nearly significant, p < 0.10); however, we did not identify any predictors for patients that required second-line neuroleptic therapy.
We reviewed 167 APCU patients who received haloperidol as a first line neuroleptic treatment for delirium. To our knowledge, this is the first study to evaluate the practice of neuroleptic rotation in patients receiving haloperidol treatment in a palliative care setting. The 65% (172/266) frequency of delirium in APCU patients in this study resembles that in a previous report (57%) [19]. Haloperidol, a neuroleptic agent with potent anti-dopaminergic properties, is still considered the drug of choice for treatment of delirium in the medically ill [10,20]. However, evidence of its effectiveness remains limited and the optimal dose has not been determined [20]. Although haloperidol cannot reverse delirium, it can reduce common symptoms of delirium including agitation, delusions, and hallucinations. Clinical trials evaluating haloperidol for delirium suggest that it is as effective as risperidone in patients with cancer-related delirium and comparable to olanzapine for management of delirium in a critical care setting [10,21,22]. Our study found that only 23% of patients (39/167) were rotated to another neuroleptic indicating that haloperidol effectively controlled delirium in a palliative care setting. The main reason of the patients needed to rotate neuroleptic were treatment failure (34/39, 87%) and adverse effects (5/39, 13%).
The success rate of second-line chlorpromazine was low (33%) in this study. This was probably due to the poor prognosis of delirium refractory to haloperidol. Accordingly, future studies to determine the best strategies for treatment of refractory delirium (e.g., rotation vs. addition vs. dose increase) are warranted.
Several previous studies reported that delirium was significantly associated with in-hospital death and poor prognosis [3,23,24]. Our group previously reported that the neuroleptic dose was significantly higher in agitated and mixed-type delirium than in patients with hypoactive delirium (p=0.008) [16]. The report of the present study did not indicate that hyperactive or mixed delirium is associated with haloperidol failure. We found that white race was another factor associated with neuroleptic rotation; however, we could not find any explanation for that finding. Language barrier might have been a possible factor. Indeed, nonwhite patients tended to have communication problems, which might have led to their receiving less aggressive care. These findings are similar to the results of our previous study of cancer pain management, in which nonwhite patients showed higher pain management barriers than white patients (p=0.02) [25].
It should be noted that this study had several limitations. First, the retrospective nature of this investigation limited the data that were collected, and some important clinical variables such as delirium subtype, and CAGE were absent from our cohort. Second, the study was confined to a single APCU in a comprehensive cancer center, which serves a unique patient population. Infrastructural and administrative parameters, clinical practices, and patient populations may vary considerably between different APCU facilities. Third, this study had a relatively small sample size.
ACKNOWLEDGMENTS
Dr. Bruera is supported in part by National Institutes of Health grants RO1NR010162-01A1, RO1CA122292-01, and RO1CA124481-01. Dr. Hui is supported in part by an institutional startup grant (#18075582). This study was also supported by the MD Anderson Cancer Center Support Grant (CA 016672). The funding sources were not involved in the conduct of the study or development of its submission. We thank Zach Bohannan in the Department of Scientific Publications at MD Anderson Cancer Center for valuable editorial assistance.
References
1. Fang CK, Chen HW, Liu SI, Lin CJ, Tsai LY, Lai YL. Prevalence, detection and treatment of delirium in terminal cancer inpatients: a prospective survey. Jpn J Clin Oncol. 2008; 38:56–63.

3. Zaal IJ, Slooter AJ. Delirium in critically ill patients: epidemiology, pathophysiology, diagnosis and management. Drugs. 2012; 72:1457–71.
4. Moyer DD. Review article: terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Care. 2011; 28:44–51.

5. Breitbart W, Alici Y. Evidence-based treatment of delirium in patients with cancer. J Clin Oncol. 2012; 30:1206–14.

6. Zhang Z, Pan L, Ni H. Impact of delirium on clinical outcome in critically ill patients: a meta-analysis. Gen Hosp Psychiatry. 2013; 35:105–11.

7. Kang JH, Shin SH, Bruera E. Comprehensive approaches to managing delirium in patients with advanced cancer. Cancer Treat Rev. 2013; 39:105–12.

9. Tatematsu N, Hayashi A, Narita K, Tamaki A, Tsuboyama T. The effects of exercise therapy on delirium in cancer patients: a retrospective study. Support Care Cancer. 2011; 19:765–70.

10. Prommer E. Role of haloperidol in palliative medicine: an update. Am J Hosp Palliat Care. 2012; 29:295–301.
11. Zimmerman K, Rudolph J, Salow M, Skarf LM. Delirium in palliative care patients: focus on pharmacotherapy. Am J Hosp Palliat Care. 2011; 28:501–10.

12. Boettger S, Friedlander M, Breitbart W, Passik S. Aripiprazole and haloperidol in the treatment of delirium. Aust N Z J Psychiatry. 2011; 45:477–82.

13. Boettger S, Breitbart W. An open trial of aripiprazole for the treatment of delirium in hospitalized cancer patients. Palliat Support Care. 2011; 9:351–7.

14. Candy B, Jackson KC, Jones L, Leurent B, Tookman A, King M. Drug therapy for delirium in terminally ill adult patients. Cochrane Database Syst Rev. 2012; 11:CD004770.

15. Hui D, Reddy A, Palla S, Bruera E. Neuroleptic prescription pattern for delirium in patients with advanced cancer. J Palliat Care. 2011; 27:141–7.

16. Hui D, Bush SH, Gallo LE, Palmer JL, Yennurajalingam S, Bruera E. Neuroleptic dose in the management of delirium in patients with advanced cancer. J Pain Symptom Manage. 2010; 39:186–96.
17. Rijcken CA, Monster TB, Brouwers JR, de Jong-van den Berg LT. Chlorpromazine equivalents versus defined daily doses: how to compare antipsychotic drug doses? J Clin Psychopharmacol. 2003; 23:657–9.

18. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991; 7:6–9.

19. Elsayem A, Mori M, Parsons HA, Munsell MF, Hui D, Delgado-Guay MO, et al. Predictors of inpatient mortality in an acute palliative care unit at a comprehensive cancer center. Support Care Cancer. 2010; 18:67–76.

20. Wang EH, Mabasa VH, Loh GW, Ensom MH. Haloperidol dosing strategies in the treatment of delirium in the critically ill. Neurocrit Care. 2012; 16:170–83.
21. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004; 30:444–9.

22. Wang HR, Woo YS, Bahk WM. Atypical antipsychotics in the treatment of delirium. Psychiatry Clin Neurosci. 2013; 67:323–31.

23. Hui D, Kilgore K, Fellman B, Urbauer D, Hall S, Fajardo J, et al. Development and cross-validation of the in-hospital mortality prediction in advanced cancer patients score: a preliminary study. J Palliat Med. 2012; 15:902–9.

Table 1.
Patient characteristics
| Characteristic | Overall (n=167) | Received second-line neuroleptic (n=39, 23%) | Did not receive second-line neuroleptic (n=128, 77%) | p-value |
|---|---|---|---|---|
| Median age (95% CI, yr) | 58 (56-61) | 57 (51-62) | 59 (57-61) | 0.4a) |
| Gender (female) | 83 (50) | 14 (36) | 69 (54) | 0.07b) |
| Race | 0.01b) | |||
| White | 113 (68) | 34 (87) | 79 (62) | |
| Black | 22 (13) | 3 (8) | 19 (15) | |
| Hispanic | 22 (13) | 1 (3) | 21 (16) | |
| Asian | 7 (4) | 0 | 7 (5) | |
| Other | 3 (2) | 1 (3) | 2 (2) | |
| ECOG | > 0.99b) | |||
| 2 | 1 (1) | 0 ( | 1 (1) | |
| 3 | 44 (26) | 10 (26) | 34 (27) | |
| 4 | 122 (73) | 29 (74) | 93 (73) | |
| Cancer | 0.20c) | |||
| Gastrointestinal | 39 (23) | 7 (18) | 32 (25) | |
| Hematologic | 29 (17) | 6 (15) | 23 (18) | |
| Lung | 27 (16) | 9 (23) | 18 (14) | |
| Breast | 19 (11) | 5 (13) | 14 (11) | |
| Gynecologic | 16 (10) | 2 (5) | 14 (11) | |
| Genitourinary | 9 (5) | 0 | 9 (7) | |
| Sarcoma | 8 (5) | 2 (5) | 6 (5) | |
| Head and neck | 7 (4) | 4 (10) | 3 (2) | |
| Other | 13 (8) | 4 (10) | 9 (7) | |
| CAGE (positive) | 48 (29) | 11 (28) | 37 (29) | > 0.99b) |
| Median APCU stay (IQR, day) | 5 (3-7) | 6(4-8) | 5 (3-7) | 0.20a) |
| Median MDAS at admission (IQR, day) | 11 (8-18) | 13 (8-26) | 10 (8-17) | 0.13a) |
| Median ESAS at admission (IQR) | ||||
| Pain | 5 (3-8) | 4.5 (3-7) | 5 (3-8) | 0.56a) |
| Fatigue | 6 (4-8) | 7 (5-9) | 6 (4-8) | 0.26a) |
| Nausea | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0.41a) |
| Depression | 3 (0-5) | 2 (0-5) | 3 (0-5) | 0.47a) |
| Anxiety | 4 (0-6) | 5 (1-8) | 3 (0-5.5) | 0.14a) |
| Drowsiness | 3.5 (2-5.5) | 3.5 (2-5) | 3.5 (1-6) | 0.89a) |
| Appetite | 5 (3-8) | 6 (5-8) | 5 (3-8) | 0.11a) |
| Wellbeing | 5.5 (4-7) | 6 (5-7) | 5 (4-8) | 0.66a) |
| Drowsiness | 3.5 (2-5.5) | 3.5 (2-5) | 3.5 (1-6) | 0.89a) |
| Sleep | 4 (2-6) | 5.5 (4-7) | 3 (2-6) | 0.07a) |
| DNR at admission (yes) | 151 (90) | 36 (92) | 115 (90) | > 0.99b) |
| Discharge status (alive) | 107 (64) | 16 (41) | 91 (71) | < 0.01b) |
| Admission type | 0.16b) | |||
| Transfer | 92 (55) | 25 (64) | 67 (52) | |
| Emergency center | 58 (35) | 13 (33) | 45 (35) | |
| Outpatient | 17 (10) | 1 (3) | 16 (13) | |
| Discharge type | 0.01b) | |||
| Home or home hospice | 52 (31) | 7 (18) | 45 (35) | |
| Inpatient hospice | 49 (29) | 8 (21) | 41 (32) | |
| Other hospital | 6 (4) | 1 (3) | 5 (4) | |
| Inpatient mortality | 60 (36) | 23 (59) | 37 (29) |
Values are presented as number (% or range). CI, confidence interval; ECOG, Eastern Cooperative Oncology Group performance status; CAGE, cut down, annoying, guilty, eye-opener; APCU, acute palliative care unit; IQR, interquartile ranges; MDAS, Memorial Delirium Assessment Scale; ESAS, Edmonton Symptom Assessment Scale; DNR, do not resuscitate.
Table 2.
Delirium characteristics and treatment in patients with and without the need for second-line neuroleptic
| Characteristic | Rotated (n=39, 23.4%) | Not rotated (n=128, 76.6%) | p-value | |
|---|---|---|---|---|
| Delirium subtype | 0.46a) | |||
| Hyperactive and mixed | 23 (59) | 63 (52) | ||
| Hypoactive | 16 (41) | 59 (48) | ||
| Median initial haloperidol dose (mg) | 6 (5-7) | 5 (4-6) | 0.12b) | |
| Overall | 5 (5-7) | |||
| Median duration of haloperidol (day) | 4 (2-6) | 5 (4-7) | 0.06b) | |
| Overall | 5.6 (5.1-6.1) | |||
| Median final haloperidol dose (mg) | 6 (5-8) | 6 (5-7) | 0.13b) | |
| Overall | 6.4 (5.9-7.0) | |||
| Improvement of delirium after first-line neuroleptic treatment (yes) | 2 (5) | 73 (57) | < 0.01a) | |
| Reason for neuroleptic rotation | - | |||
| Non-efficacy | 34 (87) | - | ||
| Adverse event | 5 (13) | - | ||
| Median initial chlorpromazine dose (mg) | 150 (100-150) | - | - | |
| Median duration of chlorpromazine (day) | 3 (2-6) | - | - | |
| Median HEDD at the final second-line neuroleptic treatment (mg) | 12 (8-16) | - | - | |
| Improvement of delirium after second-line neuroleptic treatment (yes) | 13 (33) | - | - | |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download