Introduction
The incidence of breast cancer is increasing in Korea, and it is now the second most common cancer in women, with an age adjusted incidence rate in 2010 of 45.4 [
1]. This increase in breast cancer incidence is partly attributed to improved screening and diagnostic techniques, as well as changes in reproductive patterns, specifically, women waiting until later to have children [
2]. In Korea, cancer screening rates have shown a significant increase from 2004 to 2012, and screening rates for breast cancers are now approaching 70% [
3].
Through achievement of remarkable advances in cancer treatment and early detection of cancer, the number of patients who survive cancer has shown a significant increase. Grundmann and Meyer [
4] suggested that rising population age and advances in treatment with improved survival from cancer have led to more frequent survival of cancer treatment and subsequently more patients with a second primary tumor. The reasons for these changes may be environmental modifications, genetic predisposition, therapy, or increased surveillance.
The criteria for diagnosing multiple primary tumors are 1) each tumor must present a definite picture of malignancy; 2) each must be distinct; and 3) the probability that one was a metastatic lesion originating from the other must be excluded. Patients with metachronous cancer are defined as those diagnosed with a secondary cancer six months or more after their primary diagnosis with breast cancer.
Although there have been numerous reports of metachronous cancers among breast cancer patients, most are from Western countries and in case report form. Additionally, the incidence of metachronous cancer varies to the extent that the clinical results differ in each study, and the general pattern of metachronous breast cancer still needs to be clarified. Further characterization of metachronous breast cancer could provide valuable information for use in early diagnosis and treatment of these diseases. Awareness, suspicion of multiple primary malignancy and aggressive diagnostic work ups play crucial roles in detection of cancer in earlier stages and can result in a better outcome.
This study was designed to evaluate the relative risk (RR) of developing additional cancers at different sites and the prognosis of metachronous breast cancer in Korea.
Go to :

Materials and Methods
Between March 1994 and July 2012, 2,657 patients underwent surgical treatment or chemotherapy for breast cancer at Keimyung University Dongsan Medical Center, excluding those receiving treatment for breast lymphoma. Patients who were diagnosed with new primary cancer developing from other organs were selected from the original group. Among the 2,657 patients who underwent treatment for breast cancer, metachronous cancers were diagnosed in 108 patients over a period of 18 years. Patients with metachronous cancer are defined as those with an interval of six months or more after their initial diagnosis of breast cancer. All patients with breast cancer were histologically diagnosed by core needle or surgical biopsy. Second primary cancer was confirmed by histological diagnosis; however, hepatocellular carcinoma was confirmed in two cases by imaging and a tumor marker study. Therefore, it is unlikely that any metastasis was mistaken as a second primary cancer. The age of the patients at the onset of metachronous cancer or in the TNM stage according to the sixth edition of the American Joint Committee on Cancer (AJCC), and the presence of factors that could mediate effects on survival rate were examined retrospectively. Evaluation of the interval from the first pathologic diagnosis of breast cancer to the development of a second primary cancer, clinical characteristics, and survival rate after diagnosis of a second cancer was based on medical records.
The control population was extracted from data provided by the Korean Central Cancer Registry and the National Cancer Center operated by the Ministry of Health and Welfare.
1. Statistical analysis
The yearly incidence (per 100,000) of the various malignancies in both the control and breast cancer were compared. The ratio of these two values and 95% confidence intervals indicated the RR coefficient of having cancer.
The Kaplan-Meier method was used for generation of overall survival curves. The survival curve for patients with metachronous double primary cancer was calculated from the date of diagnosis of the second cancer. Analyses were conducted using SPSS ver. 18.0 (SPSS Inc., Chicago, IL).
Go to :

Results
Among 2,657 patients with breast cancer, 108 (4.1%) were diagnosed with metachronous double primary cancer. In a comparison of the sex distribution of the patients, double primary cancer occurred more frequently in males but was not statistically different. Double primary cancer occurred in one of 15 males (6.7%) and 107 of 2,642 female (4.2%) patients (p=0.464). As shown in
Table 1, age distribution between groups did not differ (p=0.053). The most common age group included patients aged 40 to 50, and the most common metachronous double primary cancer was thyroid cancer, followed by stomach, endometrium, cervix, and lung cancers.
Table 1.
Sex and age distribution at the time of diagnosis of breast cancer with or without second primary cancer
|
Age (yr) |
With second cancer
|
Without second cancer
|
|
Male |
Female |
Male |
Female |
|
11-20 |
- |
- |
0 |
3 (0.1) |
|
21-30 |
- |
- |
0 |
61 (2.4) |
|
31-40 |
0 |
6 (5.6) |
1 (7.1) |
354 (14.0) |
|
41-50 |
0 |
43 (40.2) |
1 (7.1) |
914 (36.1) |
|
51-60 |
0 |
35 (32.7) |
4 (28.6) |
675 (26.6) |
|
61-70 |
1 |
16 (15.0) |
6 (42.9) |
360 (14.2) |
|
71-80 |
0 |
6 (5.6) |
2 (14.3) |
144 (5.7) |
|
81-90 |
0 |
1 (0.9) |
0 |
24 (0.9) |
|
Total |
1 |
107 |
14 |
2,535 |

Table 2 shows the median age distribution of patients with metachronous double primary cancer. A significant difference was observed among the types of metachronous double primary cancers (p < 0.001), and the median age was 55.9±10.3 years. Hepatocellular cell carcinoma had the oldest median age of 73.8 years, while nasopharyngeal cancer had the youngest age of 39.5 years.
Table 2.
Cancer type and mean age distribution of metachronous double primary cancer in breast cancer patients
|
Cancer type |
Double primary cancer
|
|
No. |
Age (yr) |
|
Thyroid cancer |
45 (41.7) |
52.0±7.3 (41.6-73.9) |
|
Stomach cancer |
16 (14.8) |
65.4±4.7 (46.8-74.3) |
|
Endometrial cancer |
10 (9.3) |
55.3±13.6 (39.3-83.3) |
|
Cervical cancer |
7 (6.5) |
48.9±15.7 (35.0-72.6) |
|
Lung cancer |
5 (4.6) |
65.0±5.3 (58.4-72.1) |
|
Rectal cancer |
4 (3.7) |
60.7±12.3 (44.6-74.6) |
|
Acute myelocytic leukemia |
4 (3.7) |
50.0±6.3 (48.1-61.8) |
|
Colon cancer |
3 (2.8) |
65.4±4.7 (59.0-68.1) |
|
Biliary cancer |
3 (2.8) |
68.7±12.8 (60.5-85.7) |
|
Ovarian cancer |
3 (2.8) |
57.3±3.3 (54.4-61.0) |
|
Hepatocellular carcinoma |
2 (1.9) |
73.8±3.3 (71.5-76.2) |
|
Bladder cancer |
2 (1.9) |
69.3±11.8 (61.0-77.6) |
|
Nasopharyngeal cancer |
1 (0.9) |
39.5 |
|
Renal cell carcinoma |
1 (0.9) |
49.5 |
|
Acute lymphocytic leukemia |
1 (0.9) |
48.2 |
|
Total |
108 |
55.9±10.3 (35.0-85.7) |

Table 3 shows the yearly diagnostic period of metachronous double primary cancer and does not indicate any notable difference in incidence from year to year. The mean diagnostic period was 58.4±41.2 months, and no significant differences were observed among cancer types (p=0.157). Acute leukemia, thyroid, colorectal, and biliary cancers were diagnosed earlier than other cancers (
Table 4).
Table 3.
Diagnostic period of metachronous double primary cancer in patients with breast cancer
|
Diagnostic period (yr) |
No. |
|
< 1 |
13 |
|
1-3 |
28 |
|
3-5 |
19 |
|
5-7 |
14 |
|
7-9 |
21 |
|
9-1 |
9 |
|
11-13 |
2 |
|
13-15 |
1 |
|
15-17 |
1 |
|
Total |
108 |

Table 4.
Mean diagnostic period of metachronous double primary cancer in breast cancer patients
|
Cancer type |
Diagnostic period (mo) |
|
Thyroid cancer |
48.9±33.9 (7.1-121.8) |
|
Stomach cancer |
71.5±41.5 (6.9-129.3) |
|
Endometrial cancer |
60.5±37.4 (8.5-107.9) |
|
Cervical cancer |
61.3±60.9 (8.5-180.2) |
|
Lung cancer |
89.1±45.9 (21.9-142.3) |
|
Rectal cancer |
53.1±58.4 (8.3-135.2) |
|
Acute myelocytic leukemia |
21.1±11.0 (11.0-36.3) |
|
Colon cancer |
51.9±63.2 (9.5-124.5) |
|
Biliary cancer |
51.3±29.5 (24.2-82.7) |
|
Ovarian cancer |
56.5±38.4 (13.2-90.4) |
|
Hepatocellular carcinoma |
86.4±49.4 (51.5-121.3) |
|
Bladder cancer |
129.65±45.5 (97.5-161.8) |
|
Nasopharyngeal cancer |
69.7 |
|
Renal cell carcinoma |
84.7 |
|
Acute lymphocytic leukemia |
26.4 |
|
Total |
58.4±41.2 (6.9-180.2) |

The occurrence of a double primary cancer within 5 years of the initial diagnosis of breast cancer was evident in 60 patients (55.6%). Ten years after the initial diagnosis of breast cancer, 10 patients (9.3%) were diagnosed with double primary cancer. These cases included stomach cancer in two patients, lung cancer in two patients and thyroid, cervical, rectal, colon, liver, and bladder cancer in one patient each. The development of hepatocellular carcinoma (50.0%), bladder cancer (50.0%), and lung cancer (40.0%) occurred much more frequently in 10 years after the diagnosis of breast cancer (
Table 5).
Table 5.
Diagnosis of metachronous double primary cancer 5 years and 10 years after diagnosis of breast cancer
|
Cancer type |
After 5 years |
After 10 years |
Total |
|
Thyroid cancer |
17 (37.8) |
1 (2.2) |
45 |
|
Stomach cancer |
9 (56.3) |
2 (12.5) |
6 |
|
Endometrial cancer |
5 (50.0) |
- |
10 |
|
Cervical cancer |
3 (42.9) |
1 (14.3) |
7 |
|
Lung cancer |
4 (80.0) |
2 (40.0) |
5 |
|
Rectal cancer |
1 (25.0) |
1 (25.0) |
4 |
|
Acute myelocytic leukemia |
- |
- |
4 |
|
Colon cancer |
1 (33.3) |
1 (33.3) |
3 |
|
Biliary cancer |
1 (33.3) |
- |
3 |
|
Ovarian cancer |
2 (66.7) |
- |
3 |
|
Hepatocellular carcinoma |
1 (50.0) |
1 (50.0) |
2 |
|
Bladder cancer |
2 (100) |
1 (50.0) |
2 |
|
Nasopharyngeal cancer |
1 (100) |
- |
1 |
|
Renal cell carcinoma |
1 (100) |
- |
1 |
|
Acute lymphocytic leukemia |
- |
- |
1 |
|
Total |
48 (44.4) |
10 (9.3) |
108 |

Table 6 shows the RR of metachronous double primary cancer in breast cancer. The RR was higher for nasopharyngeal (6.30), endometrial (4.78), acute myelogenous leukemia (2.61), stomach (2.61), bladder (2.37), and thyroid cancer (1.95) with primary breast cancer, but only endometrial, gastric and thyroid cancer were statistically significant.
Table 6.
Relative risk of metachronous double primary cancer in breast cancer patients
|
Cancer type |
Relative risk |
95% CI |
|
Nasopharyngeal cancer |
6.30 |
0.15-271.59 |
|
Endometrial cancer |
4.78 |
1.66-13.79 |
|
Acute myelocytic leukemia |
2.61 |
0.70-9.81 |
|
Stomach cancer |
2.61 |
1.68-4.06 |
|
Bladder cancer |
2.37 |
0.39-14.40 |
|
Thyroid cancer |
1.95 |
1.37-2.79 |
|
Ovarian cancer |
1.05 |
0.33-3.34 |
|
Biliary cancer |
1.04 |
0.33-3.28 |
|
Cervical cancer |
0.94 |
0.45-1.95 |
|
Acute lymphocytic leukemia |
0.88 |
0.13-5.94 |
|
Lung cancer |
0.87 |
0.40-1.91 |
|
Renal cell carcinoma |
0.81 |
0.12-5.24 |
|
Rectal cancer |
0.55 |
0.23-1.32 |
|
Colon cancer |
0.35 |
0.14-0.90 |
|
Hepatocellular carcinoma |
0.34 |
0.11-1.08 |
|
Total |
0.95 |
0.79-1.15 |

The mean follow-up period was 45.9 months for metachronous cancers. The mean survival time of metachronous double primary cancer was 169.1±9.8 months (95% confidence interval [CI], 149.8 to 188.3 months) (
Fig. 1). The 5-year survival rate and median survival of metachronous cancer in breast cancer patients was 72.3% and 123.9±11.2 months, respectively. The 5-year survival rates after diagnosis of second cancer were 100% for thyroid and colon cancer, 83.3% for cervical cancer, 80.0% for endometrial cancer, 66.7% for rectal cancer, 61.9% for stomach cancer, 22.2% for lung cancer, and 0% for liver, biliary, ovarian cancer and acute leukemia, and these values differed significantly according to cancer type (p < 0.001) (
Table 7). The median survival period for patients with endometrial, cervical, and colorectal cancers was similar to or longer than that of other cancers.
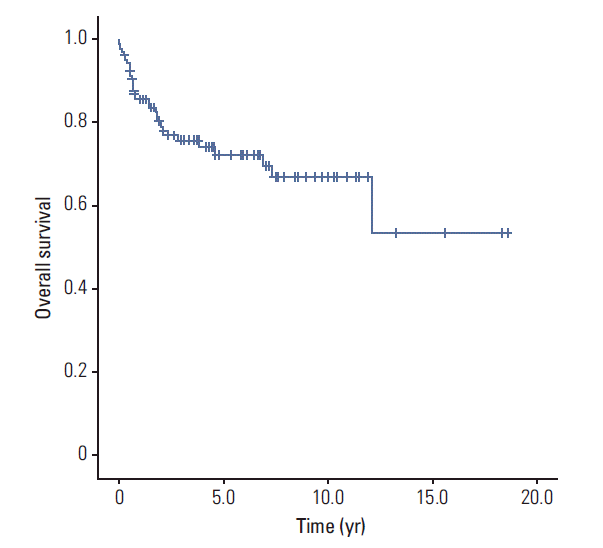 | Fig. 1.Kaplan-Meier survival curve of metachronous double primary cancer of breast cancer patients from the diagnosis of second cancer (n=108). 
|
Table 7.
Median survival after diagnosis of metachronous double primary cancer in patients with breast cancer
|
Cancer type |
No. |
5-Year survival rate (%)a)
|
Median±SD (mo) |
95% CI |
|
Thyroid cancer |
45 |
100 |
- |
- |
|
Stomach cancer |
16 |
61.9 |
64.4±12.4 |
40.2-88.7 |
|
Endometrial cancer |
10 |
80.0 |
150.3±22.8 |
105.6-195.0 |
|
Cervical cancer |
7 |
83.3 |
128.4±29.8 |
69.9-186.9 |
|
Lung cancer |
5 |
22.2 |
18.0±4.4 |
9.3-26.7 |
|
Rectal cancer |
4 |
66.7 |
85.9±26.8 |
33.5-138.4 |
|
Acute myelocytic leukemia |
4 |
0 |
3.2±1.6 |
0.1-6.3 |
|
Colon cancer |
3 |
100 |
121.4 |
- |
|
Biliary cancer |
3 |
0 |
14.8±6.1 |
2.9-26.7 |
|
Ovarian cancer |
3 |
0 |
36.6±10.9 |
15.3-57.9 |
|
Hepatocellular carcinoma |
2 |
0 |
10.4±3.8 |
3.0-17.8 |
|
Bladder cancer |
2 |
- |
6.8 |
- |
|
Nasopharyngeal cancer |
1 |
- |
- |
- |
|
Renal cell carcinoma |
1 |
- |
- |
- |
|
Acute lymphocytic leukemia |
1 |
0 |
- |
- |
|
Total |
108 |
72.3 |
123.9±11.2 |
102.0-145.9 |

Go to :

Discussion
Multiple primary cancers seem to be increasing in frequency, but various quantitative differences have been observed. For example, cancer patients in Connecticut had a 31% higher risk of developing a subsequent cancer [
5], whereas no excessive risk was observed in Denmark [
6] or Finland [
7]. Differences in study methodology may explain these apparent discrepancies in part, but it is possible that they are due to common genetic susceptibility and environmental factors such as etiological aspects (e.g., for smoking or diet-related cancers) and/or different consequences of cancer treatment in various populations.
In this study, the incidence of second primary cancer was 4.1% and the RR of all cancer in breast cancer was 0.95. This figure appears to be underestimated, as patients who had not been diagnosed with pathologic confirmation due to their physical condition and/or having difficulty in receiving a pathologic diagnosis were excluded. It should be noted that this study had a relatively limited study population size and excluded secondary breast cancer patients, which likely led to underestimation of the total incidence of patients with a second primary cancer. In another Korean study [
8], the incidence rate of metachronous cancer was 34 in 7,735 breast cancer patients (0.4%), which was unusually low when compared with present hospita l-based cancer registry studies. Raymond and Hogue [
2] found that 12.3% of breast cancer survivors were diagnosed with second primary malignant tumors, while Schenker et al. [
9] found that figure to be 8% in a similar study. Mellemkjaer et al. [
10] found a standardized incidence rate (SIR) of total metachronous primary cancer in breast cancer of 1.25, while the SIR was 1.2 and 1.7 for total metachronous cancer and second breast cancer, respectively, in the Swiss Vaud Cancer Registry [
11]. These findings suggest that second breast cancer is a high component of second primary cancer. However, Stracci et al. [
12] investigated data from the Umbrian Population Cancer Registry (RTUP) and found that the SIR was non-significant, including that for metachronous contralateral breast cancer.
In this study, the most common second double primary cancer was thyroid cancer, followed by stomach, endometrial, cervical, and lung cancers. The statistically significant cancers were endometrial cancer (RR, 4.78), stomach cancer (RR, 2.61), and thyroid cancer (RR 1.95). Breast cancer survivors had the greatest risk for developing breast, bone, colon/rectal, connective tissue (sarcoma), leukemia, lung, ovary, or thyroid cancer [
2]. Schenker et al. [
9] demonstrated that the most common second primary cancer in breast cancer patients was that of the opposite breast (23.9%), and that the diagnosis of second breast cancer was made an average of 7 years after the first cancer was detected. Their study also revealed that the likelihood of developing uterine corpus, thyroid and ovarian cancer was greater than in the general population, while stomach and gall bladder cancer had relatively low risks (0.5 and 0.3, respectively). Mellemkjaer et al. [
10] found the following SIR rates for second cancer: stomach 1.35, colorectal 1.22, lung 1.24, soft tissue sarcoma 2.25, melanoma 1.29, non-melanoma skin 1.58, endometrium 1.52, ovary 1.48, kidney 1.27, thyroid 1.62, and leukemia 1.52. In the Swiss Vaud Cancer registry, the SIR was 2.2 for thyroid cancer, 1.4 for pancreas cancer, 1.2 for uterine corpus cancer, and 1.1 in colorectal cancer [
11]. Stracci et al. [
12] reported an increased occurrence of salivary gland, uterine corpus, ovarian and thyroid cancer, as well as a significant excess of melanoma and second breast cancers. The excessive skin melanoma in breast cancer survivors was attributed to the relationship with
BRCA2 and
CDKN2A mutation-positive patients. Parikh and Advani [
13] conducted a study in India that revealed the most prevalent second tumor to be ovarian cancer, and that second cancer patients have worse survival rates than breast cancer patients without second cancer. The incidence of metachronous second cancer varies among studies, and the discrepancies in the type of second cancer is influenced by several factors including race, hormone status, environment, and genetic disposition. Studies have provided consistent findings in terms of excesses of various cancer sites for cancer of the endometrium [
7,
10,
14], thyroid cancer [
2,
7,
9-
12,
14,
15], and ovarian cancer [
2,
9,
10,
12,
13,
16,
17]. Breast, ovary, and endometrial cancers may have a common etiology, with high fat diet, effect of sex hormone levels, and reduced fertility being candidate factors [
9].
The mean diagnostic period of second primary cancer was 58.4±41.2 months, while it was 46.4 months in another Korean study [
4]. The diagnostic period showed no significant differences among cancer types (p=0.157). Acute leukemia, thyroid, colorectal and biliary cancers were diagnosed earlier than other cancers (
Table 4). Five years after the initial diagnosis of breast cancer, a second primary cancer was diagnosed in 48 patients (44.4%). After 10 years, 10 patients (9.3%) were diagnosed with a double primary cancer. Clinicians previously believed that being cancer free for 5 years after the initial diagnosis indicated that the patient was cured of cancer, and the results of the present study indicate that a considerable proportion of women who developed a second cancer were cancer-free 5 years after their initial diagnosis.
Of all patients who developed a second primary cancer following cancer of the breast, a significantly high rate of previous radiotherapy was only found in those with lung cancer (82%) and cancer of the hematopoietic system (95%) [
9]. The cancer types of concern with respect to radiotherapy are those in organs close to the breast, such as the esophagus, lung, thyroid gland, stomach, soft tissue sarcoma of the thorax and upper limb and leukemia [
7]. Thyroid cancer is regarded as a radiation inducible cancer, as excess cancer of the thyroid is not treatment induced [
10]. Grundmann and Meyer [
4] proposed that radiotherapy increases the rate of second primary neoplasm, and that adjuvant radiotherapy should be well justified. Nevertheless, this is true only for young patients, mainly in childhood, and the risk of a second cancer after irradiation in adults is small. In this study, radiation treatment was only administered to 37.8% of second thyroid cancer patients.
Tamoxifen may be associated with endometrial proliferation, hyperplasia, polyp formation, invasive carcinoma, and uterine sarcoma. An increased risk of endometrial cancer has been observed in prevention and adjuvant trials of tamoxifen [
18]. Mellemkjaer et al. [
10] suggested that excess endometrial cancer was not entirely caused by tamoxifen. Thus, common risk factors such as reproductive and genetic factors, obesity, and/or some unknown factors are likely responsible for some of this excess. Any symptoms of endometrial hyperplasia or cancer reported by postmenopausal women taking tamoxifen should be evaluated. Premenopausal women treated with tamoxifen have no known increased risk of uterine cancer, and therefore require no additional monitoring beyond routine gynecologic care. If a typical endometrial hyperplasia develops, appropriate gynecologic management should be instituted, and the use of tamoxifen should be reassessed. The American Society of Clinical Oncology (ASCO) Panel recommends that women with intact uteri who are taking adjuvant tamoxifen have annual gynecologic assessments and undergo rapid evaluation of any vaginal spotting that might occur because of the risk of tamoxifen-associated endometrial carcinoma in postmenopausal women [
19]. However, routine endometrial biopsy or ultrasonography of asymptomatic women is not recommended because the vast majority of women with tamoxifen-associated uterine carcinoma have early vaginal spotting [
19]. In this study, 10 endometrial cancer patients received tamoxifen, and vaginal spotting was observed in 30% of those diagnosed in the early stage through regular, periodic gynecologic examination. The 5-year survival rate found in the study (80.0%) was similar to that of the occurrence in the general population (86.2%).
In this study, the 5-year survival and median survival rates of metachronous cancer in breast cancer patients were 72.3% and 123.9±11.2 months, respectively. This study also found significantly different 5-year survival rates after the diagnosis of second cancer of 100%, 83.3%, 80.0%, 66.7%, and 61.9% in thyroid and colon, cervical, endometrial, rectal, and stomach cancer patients, respectively (p < 0.001). Endometrial, cervical and colon cancers had a high median survival due to early detection through annual periodic examination. In general, overall survival was poorer for women with multiple primary tumors than women with no second tumors [
2], and cancers known to be associated with low survival rates when appearing on their own have the same effect when they occur in breast cancer patients [
9]. The prognosis of breast cancer patients with multiple primary tumors depends on the type of the second primary tumor, its malignancy, and its response to treatment.
Go to :






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download