Abstract
Purpose
Capecitabine is known to increase mean corpuscular volume (MCV). To define the incidence of capecitabine-induced macrocytosis and its association with chemotherapy outcomes, we investigated data of 89 patients with advanced gastric cancer (AGC) who were enrolled in a randomized chemotherapy trial involving capecitabine.
Materials and Methods
Chemotherapy-naïve AGC patients were treated with capecitabine (1,000 mg/m2/day on days 1-14) plus cisplatin (75 mg/m2 on day 1), with or without epirubicin (50 mg/m2 on day 1). Complete blood counts including MCV were measured at baseline and on day 1 of each 3-week chemotherapy course. Macrocytosis was defined as a MCV increase > 10 fL from baseline. Multivariate Cox proportional hazards models were used for analysis of the impact of clinical and MCV values on chemotherapy outcomes.
Results
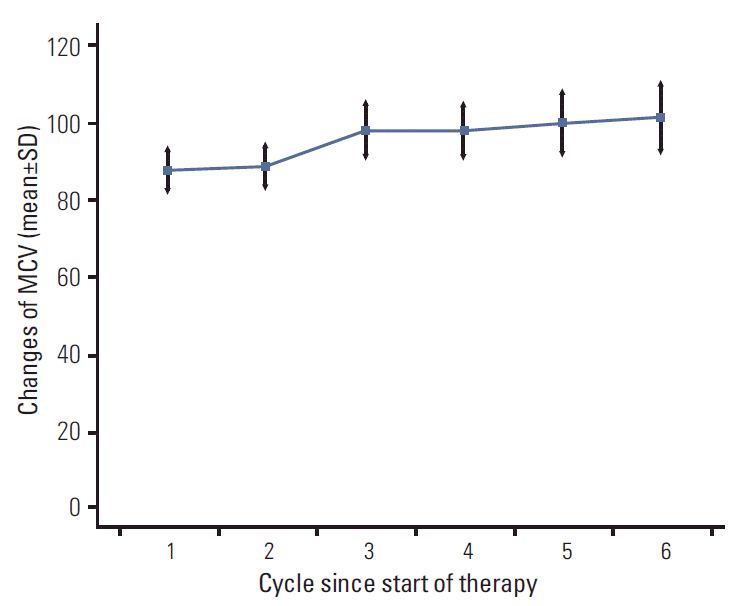
At baseline, the mean MCV was 88.2 fL (normal range, 80 to 100 fL). During chemotherapy, MCV increased in a dose-dependent manner with a mean increase of 11.3 fL. MCV elevation after capecitabine treatment in 74 patients (90%) and 44 patients (42%) developed macrocytosis.
Results
of multivariate analysis showed that development of macrocytosis was independent of baseline hemoglobin level, liver metastasis, performance status, or liver function. The number of chemotherapy cycles showed strong association with development of macrocytosis and hematologic adverse events. In addition, a significant association was observed between macrocytosis and clinical response or survival.
Capecitabine (Xeloda, F. Hoffmann La-Roche, Basel, Switzerland) is an oral fluoropyrimidine designed to mimic continuous infusion of 5-fluorouracil (5-FU). Capecitabine is considered to be more effective and tolerable than 5-FU [1], and has been shown to exhibit antitumor activity against a variety of gastrointestinal tumors, including gastric cancer [2]. In addition to efficacy, the patient’s convenience and satisfaction are assuming increasing importance in cancer management. Oral administration has the advantage of allowing convenient patient-orientated therapy, providing the patient with a degree of independence and improved quality of life while avoiding complications associated with intravenous drug administration [3]. The toxicity profile of capecitabine is generally described as manageable, with hand-foot skin reaction being the main toxicity [4].
In addition to hand-foot skin reaction, retrospective studies have indicated that the use of capecitabine has a significant influence on the mean corpuscular volume (MCV) [5-7]. Treatment with any drug that affects cellular DNA biosynthesis either directly or indirectly may result in macrocytic changes. An elevated MCV is an expression of DNA biosynthetic changes [8]. Certain chemotherapeutic agents that have 5-FU as their principal representative, such as capecitabine, also interfere with DNA synthesis and may cause macrocytosis with or without megaloblastic changes. Vrecl et al. [9] reported that MCV showed a gradual increase in all patients receiving capecitabine, beginning to increase within the first two cycles of therapy and normalizing after cessation of capecitabine treatment.
One might argue that development of macrocytosis could have prognostic or predictive significance for chemotherapy response or survival. The underlying assumption is that macrocytosis is caused by inhibition of thymidylate synthase (TS) in erythroid precursor cells and TS expression is known to be associated with the outcome of patients receiving fluoropyrimidine therapy [10]. If capecitabine-induced macrocytosis is predictive of survival or tumor response in patients with advanced gastric cancer (AGC), simple serial measurement of MCV may be a practical and non-invasive method of identifying patients who would benefit from capecitabine or those who need to consider changing to other agents.
We previously reported a prospective, randomized trial comparing two capecitabine-containing combination regimens as first-line palliative chemotherapy in patients with AGC [11]. To better define the incidence of capecitabineinduced macrocytosis and its association with treatment outcomes, we examined clinical and laboratory data of all patients enrolled in the trial.
We reviewed the records of 89 patients with chemotherapy-naïve AGC (recurrent metastatic gastric cancer) who were treated with capecitabine-containing combination chemotherapy. Details on patient eligibility and treatment were previously reported [11]. In brief, patients were randomly assigned to receive 3-week cycles of cisplatin and capecitabine (CX), or CX plus epirubicin (ECX). In both arms of the study, capecitabine was administered orally at a dose of 1,000 mg/m2 twice daily as an intermittent regimen of two weeks of treatment followed by one week of rest. Chemotherapy was repeated every three weeks until unacceptable toxicities or evidence of disease progression were seen. Patients were required to give written informed consent before inclusion in the study. The study was approved by the institutional review board of Samsung Medical Center (Seoul, Korea), and conducted in accordance with the principles of the Declaration of Helsinki. Supportive care including administration of blood transfusions, and use of hematopoietic growth factors, antiemetics, and analgesics was permitted if judged appropriate by the investigators.
Laboratory tests including complete blood counts were checked at baseline, and on day 1 of each 3-week course of chemotherapy. Toxicities were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver. 3. The primary endpoint was duration of overall survival (OS), which was calculated from the day of randomization to the date of death by any cause. Patients who were alive on the last day of follow up were censored. The median follow-up duration for all patients was 12.4 months (95% confidence interval [CI], 11.6 to 13.1 months). The secondary endpoints of this study were progression-free survival (PFS) and clinical response, which was evaluated every two cycles according to the Response Evaluation Criteria in Solid Tumors (RECIST) [12] and was assessed by abdominopelvic computed tomography or by the same tests that were initially used to stage the tumor.
The analysis investigated the predictive or prognostic value of the development of macrocytosis, defined as an increase in MCV > 10 fL measured during treatment, and the following clinical parameters: age, gender, performance status (PS), treatment arm, presence of peritoneal dissemination, baseline hemoglobin level, number of chemotherapy cycles, and cumulative dose of capecitabine. Associations between the possible predictors and the dependent variables of survival duration and tumor response were determined using Cox proportional hazards regression models. The proportionality of hazards assumption for the effect of increased MCV was satisfied by examining it as a timedependent parameter in the model. Tests of interaction between development of macrocytosis and potentially modifying baseline parameters were assessed by entering the cross product of macrocytosis and the parameter of interest. The sample size for the current study was determined by the original study [11], which had 90% power for detection of a difference in response rate (RR) of 40% or 60%. All p-values were two-sided, with p < 0.05 indicating statistical significance. Statistical analyses were performed using the R for Windows v2.11.1 software (R Core Team, Vienna, Austria; http://www.Rproject.org).
The characteristics of the 89 patients are summarized in Table 1. The median age was 55 years (range, 33 to 75 years). Most patients had a good PS (PS of 0 or 1). Fourteen patients (16%) had hemoglobin levels of 10 g/dL or lower at baseline. Vitamin B12 and folate levels were evaluated in 10 patients, and, among these, six patients with macrocytosis had vitamin B12 and folate levels within the normal range (median vitamin B12 level, 918.5 pg/mL; range, 548 to 1,970 pg/mL [normal range, 200 to 950 pg/mL]; median folate level, 8.55 ng/mL; range, 4.1 to 11.2 ng/mL [normal range, 3 to 17 ng/mL]). Forty-five patients were treated with CX and 44 with ECX. A total of 241 CX (median, 6; range, 1 to 12) and 201 ECX (median, 5; range, 1 to 11) cycles were delivered. For patients treated with CX, the median dose intensity of capecitabine of 7,747 mg/m2/wk corresponded to 83% of the scheduled dose, and the median duration of therapy was 4.4 months (95% CI, 3.8 to 4.9 months). In the ECX arm, the median dose intensity of capecitabine was 8,000 mg/m2/wk (86% of the scheduled dose) and the median treatment duration was 4.2 months (95% CI, 3.4 to 5.0 months). No relevant difference in the overall toxicity profile or therapeutic efficacy with respect to RR, PFS, and OS was observed between the two arms.
During chemotherapy, MCV increased in a dose-dependent manner, with a mean increase of 11.3 fL (Fig. 1). At baseline, the mean MCV was 87.5 fL (standard deviation [SD], 0.8 fL). The mean MCV increased slightly to 88.5 fL (SD, 0.7 fL) before the start of the second cycle, and increased up to 98.9 fL (SD, 0.9 fL). In 44 (42%) patients, the MCV increased by more than 10 fL (i.e., macrocytosis). When the patients were stratified according to development of macrocytosis, no differences were observed in baseline characteristics, including age, weight loss, PS, number of metastatic sites, peritoneal dissemination, liver metastasis, and treatment arm (Table 1). In contrast, the number of chemotherapy cycles and cumulative capecitabine dose showed strong association with the increase in MCV. Patients who developed macrocytosis received a median number of six cycles (range, 3 to 11), whereas patients without macrocytosis received three cycles (range, 1 to 6; p < 0.001). The association of macrocytosis with duration of chemotherapy also showed statistical significance (4.5 months vs. 2.4 months; p < 0.001). Of particular interest, although capecitabine dose intensity did not differ between patients with and without macrocytosis (p=0.545), development of macrocytosis showed significant association with development of grade 3 or 4 anemia (p=0.231), thrombocytopenia (p=0.022), and neutropenia (p=0.034) during treatment.
In multivariate analysis of factors associated with development of macrocytosis, only the number of chemotherapy cycles had independent adverse significance (odds ratio, 4.310; 95% CI, 1.242 to 14.975; p < 0.001).
In the clinical trial [11], all patients were evaluable for response, except for one ECX patient. The RR was 38% and 37% in the CX and ECX arms, respectively. RR was not significantly influenced by age, gender, weight loss, PS, peritoneal dissemination, liver metastasis, or treatment arm. However, patients who developed macrocytosis during chemotherapy were more likely to respond to chemotherapy (47%) than those without macrocytosis (35%), and the difference was statistically significant (p=0.017).
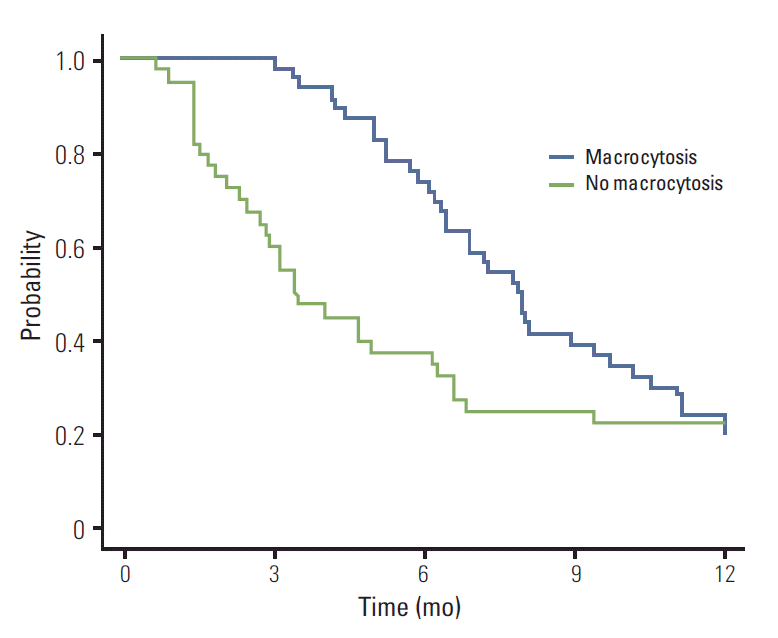
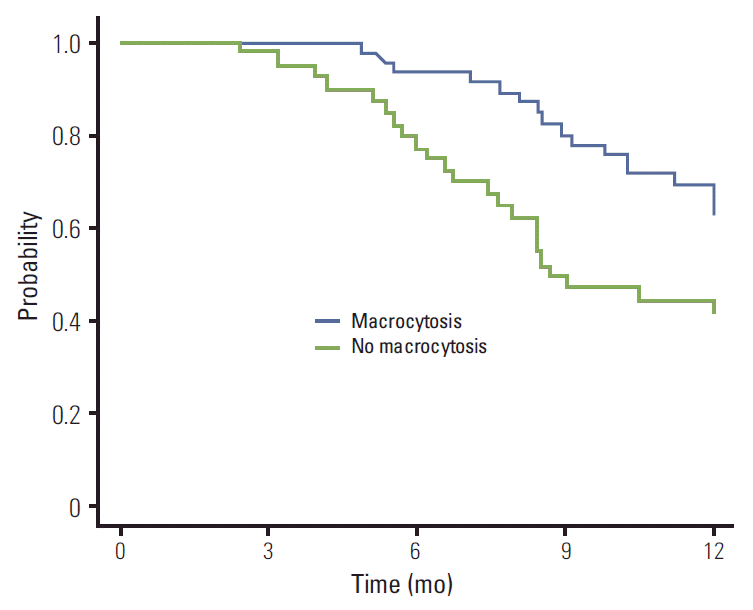
The estimated median OS and PFS were 14.3 months (95% CI, 11.4 to 17.1 months) and 6.4 months (95% CI, 5.5 to 7.3 months), respectively. Clinically relevant differences in PFS (p=0.053) or OS (p=0.033) were observed between patients who developed macrocytosis and those who did not. Patients who developed macrocytosis during treatment showed PFS and OS of 7.8 months and 14.6 months, respectively, whereas those who did not develop macrocytosis showed PFS and OS of 3.4 months and 8.7 months, respectively. The Kaplan-Meier estimates of PFS and OS are shown in Figs. 2 and 3. In the Cox proportional hazards model, previous gastrectomy, weight loss, and development of macrocytosis were independent prognostic parameters for PFS and OS. These data are summarized in Table 2. This model suggests that patients who develop macrocytosis have a risk of progression and death 1.7 and 2.1 times lower than those of patients without macrocytosis.
This study has clinical significance because it is the first study to report on the prognostic relevance of an elevation in MCV in patients with AGC who were treated with capecitabine. Using multivariate analyses we demonstrated that outcomes for RR, PFS, and OS were significantly better in patients who developed macrocytosis. Although development of macrocytosis occurred in a dose-dependent manner, baseline characteristics including age, gender, and PS were not associated with the risk of developing macrocytosis. Our results also showed an association of development of macrocytosis with the risk of developing severe hematologic toxicities, including neutropenia, anemia, and thrombocytopenia, however, no association was observed between non-hematologic toxicities and macrocytosis. This may in part be explained by the fact that higher plasma levels of capecitabine and its metabolites would also affect the probability of developing other hematologic toxicities. However, this should be further investigated in future studies because capecitabine was administered in combination with other cytotoxic agents in the current study.
Anemia, with or without treatment, is common among cancer patients. A number of factors contribute to the high incidence of cancer-related anemia. These not only include chemotherapy and radiation-induced myelosuppression, but also bleeding, hemolysis, marrow infiltration by tumor, nutritional deficiencies, and cytokine-mediated anemia of chronic disease. We already reported that anemia is common in AGC patients treated with 5-FU-based chemotherapy [13] and demonstrated a strong association between anemia and reduced RR, PFS, and OS. In cancer patients, we usually find normocytic normochromic or microcytic hypochromic types of anemia. However, if a patient receives capecitabine, one would expect an increased MCV due to capecitabineinduced inhibition of TS in erythroid precursor cells. In a report by Karvellas et al. [5], 57% of patients developed macrocytosis during capecitabine treatment and the MCV increased in a dose-dependent and time-dependent manner. In another study [7], the authors reported an association between an increase in MCV and clinical response or stable disease after capecitabine therapy.
The primary problem in predicting chemotherapy outcomes based on development of macrocytosis is that the mechanism underlying the increased MCV is not fully understood. As discussed above, capecitabine-induced macrocytosis is considered a result of TS inhibition in erythroid precursor cells, however, the data indicate that high expression of TS in tumor tissues might be associated with a significantly worse disease-free or OS [14]. Although development of macrocytosis after capecitabine therapy may be prognostic or predictive, it is still unclear whether a higher dose of capecitabine can directly impact MCV levels and/or treatment outcome. This may point toward new prospective studies or therapeutic strategies involving capecitabine based on development of macrocytosis.
Our study had the limitations associated with a retrospective study. In addition, we did not evaluate factors related to macrocytosis, such as vitamin B12 and folic acid in all patients and we could not directly compare MCV in patients treated with capecitabine and those treated with other chemotherapy regimens. However, irrespective of whether or not patients received chemotherapy, normocytic normochromic anemia is the most common type of anemia in cancer patients and macrocytic anemia is usually associated with vitamin B12 or folate deficiency. In our study, six patients who developed macrocytosis during treatment with a capecitabine containing regimen had normal levels of vitamin B12 and folate.
In conclusion, treatment with capecitabine in patients with AGC results in an increased MCV, and development of macrocytosis (increase in MCV greater than 10 fL) is significantly associated with favorable OS, PFS, and RR. Conduct of further studies testing individual dose optimization of capecitabine according to development of macrocytosis may therefore be warranted.
References
1. Twelves C. Can capecitabine replace 5-FU/leucovorin in combination with oxaliplatin for the treatment of advanced colorectal cancer? Oncology (Williston Park). 2002; 16(12 Suppl No. 14):23–6.
2. Okines A, Chau I, Cunningham D. Capecitabine in gastric cancer. Drugs Today (Barc). 2008; 44:629–40.

3. Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, Sulkes A, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer. 2002; 38:349–58.
4. Midgley R, Kerr DJ. Capecitabine: have we got the dose right? Nat Clin Pract Oncol. 2009; 6:17–24.

5. Karvellas CJ, Sawyer M, Hamilton M, Mackey JR. Effect of capecitabine on mean corpuscular volume in patients with metastatic breast cancer. Am J Clin Oncol. 2004; 27:364–8.

6. Sun JF, Wu RR, Norris C, Noone AM, Amankwa-Sakyi M, Slack R, et al. Safety of chronic low-dose capecitabine as maintenance therapy in gastrointestinal cancers. Gastrointest Cancer Res. 2009; 3:134–40.
7. Wenzel C, Mader RM, Steger GG, Pluschnig U, Kornek GV, Scheithauer W, et al. Capecitabine treatment results in increased mean corpuscular volume of red blood cells in patients with advanced solid malignancies. Anticancer Drugs. 2003; 14:119–23.

9. Vrecl R, Gerold M, Melzer S, Rainer W, Weitzer W. Effect of capecitabine on mean corpuscular volume of red blood cells. Am J Clin Oncol. 2005; 28:534.

10. van Triest B, Pinedo HM, Blaauwgeers JL, van Diest PJ, Schoenmakers PS, Voorn DA, et al. Prognostic role of thymidylate synthase, thymidine phosphorylase/platelet-derived endothelial cell growth factor, and proliferation markers in colorectal cancer. Clin Cancer Res. 2000; 6:1063–72.
11. Yun J, Lee J, Park SH, Park JO, Park YS, Lim HY, et al. A randomised phase II study of combination chemotherapy with epirubicin, cisplatin and capecitabine (ECX) or cisplatin and capecitabine (CX) in advanced gastric cancer. Eur J Cancer. 2010; 46:885–91.

12. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.
Table 1.
Baseline characteristics according to development of macrocytosis (MCV increase greater than 10 fL)
Table 2.
Multivariate analysis for OS and PFS




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download