Abstract
Purpose
The purpose of this study was to assess the value of ovarian ablation using goserelin in premenopausal patients with stage II/III hormone receptor-positive breast cancer without chemotherapy-induced amenorrhea (CIA).
Materials and Methods
We retrospectively reviewed the data of breast patients treated between October 1999 and November 2007 without CIA. The Kaplan-Meier method was used for calculation of the survival rate. Log rank method and Cox regression analysis were used for univariate and multivariate prognostic analysis.
Results
The median follow-up period was 61 months. Initially, 353 patients remained without CIA after chemotherapy and 98 among those who received goserelin and tamoxifen (TAM). In univariate analysis, goserelin improved locoregional recurrence-free survival (LRFS) (98.9% vs. 94.1%, p=0.041), distant metastasis-free survival (DMFS) (85.4% vs. 71.9%, p=0.006), disease-free survival (DFS) (85.4% vs. 71.6%, p=0.005), and overall survival (OS) (93.5% vs. 83.5%, p=0.010). In multivariate analysis, goserelin treatment was an independent factor influencing DMFS (hazard ratio [HR], 1.603; 95% confidence interval [CI], 1.228 to 2.092; p=0.001), DFS (HR, 1.606; 95% CI, 1.231 to 2.096; p=0.001), and OS (HR, 3.311; 95% CI, 1.416 to 7.742; p=0.006). In addition, treatment with goserelin resulted in significantly improved LRFS (p=0.039), DMFS (p=0.043), DFS (p=0.036), and OS (p=0.010) in patients aged < 40 years. In patients aged ≥ 40 years, goserelin only improved DMFS (p=0.028) and DFS (p=0.027).
Breast cancer is one of the most common malignancies in women. Its incidence is increasing in China, and Chinese patients with breast cancer are younger compared to their Western counterparts [1]. Although a younger age at diagnosis is associated with a poorer prognosis, more than half of these patients are positive for hormone receptor and are therefore suitable for endocrine therapy [2,3]. Both the estrogen receptor (ER) and progesterone receptor (PR) play important roles in progression of breast cancer in hormone receptor-positive patients. Thus, anti-estrogen therapy has become an important strategy for treatment of hormone receptor-positive breast cancer.
Before menopause, up to 90% of hormones are produced by the ovary in breast cancer patients [4]. Thus, ovarian ablation has become an important part of endocrine therapy and has been widely accepted in treatment of breast cancer since 1896 [5]. However, with the development of adjuvant therapy for breast cancer, there has been less emphasis on ovarian ablation. With the introduction of medical ovarian ablation using luteinizing hormone releasing hormone-agonists (LHRH-agonists), ovarian ablation with LHRH-agonists has attracted increasing attention due to its ability to reversibly suppress estrogen secretion by the ovary. Studies have shown that ovarian ablation improves the survival of premenopausal breast cancer patients who are hormone receptor-positive and have received adjuvant therapy or palliative care [6,7]. The best method for performing ovarian ablation is still controversial [6,8,9]. The current study evaluated the role of goserelin (a LHRH-agonist) in treatment of premenopausal breast cancer patients who were hormone receptor-positive and had no chemotherapyinduced amenorrhea (CIA) after chemotherapy and radiotherapy. The aim of this study was to investigate a new endocrine therapy for premenopausal women with breast cancer.
Breast cancer patients were recruited from Sun Yat-sen University Cancer Center from October 1999 to November 2007. Inclusion criteria were as follows: 1) female patients with unilateral breast cancer who had no distant metastasis at initial diagnosis; 2) patients who had undergone mastectomy and axillary lymph node dissection; 3) patients who were pathologically diagnosed with breast cancer at pT1-4N1-3 or pT3-4N0 (stage II-III) according to the 2009 Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) staging system, and postoperative immunohistochemistry showed that these patients were ER and/or PR positive; 4) patients who had received chemotherapy for at least four cycles with or without neoadjuvant chemotherapy; 5) patients who had received postoperative radiotherapy to the ipsilateral chest wall, supraclavicular and infraclavicular region; 6) patients who had received regular endocrine therapy; and 7) patients who had regular menstrual cycles or temporary amenorrhea (the resumption of menstruation after at least three months of amenorrhea) after chemotherapy, or levels of estradiol, follicle-stimulating hormone (FSH), and luteinizing hormone similar to premenopausal levels. Approval for retrospective analysis of the patient data was obtained from the ethics committee of Sun Yat-sen University Cancer Center.
CIA was defined as the cessation of menstruation for > 6 consecutive months. Resumption of menstruation was defined as regular cyclic bleeding after CIA for > 3 months without pathologic etiology. Serum estradiol (E2) and FSH were tested using chemiluminescence immunoassay once every month. Premenopausal hormone levels of E2 ≥ 110 pmol/L and FSH ≤ 21.7 IU/L were also defined as without CIA regardless of menstrual bleeding.
Treatment with ovarian ablation using goserelin was recommended by the clinician but was to be according to patients’ decision. Patients who underwent LHRH-agonist therapy received a subcutaneous depot injection of goserelin (Zoladex, AstraZeneca, London, UK) at 3.6 mg once every month (four weeks) for at least two years according to the recommendation of the European Society for Medical Oncology (ESMO) and The Adjuvant Breast Cancer Ovarian Ablation or Suppression Trial [10,11]. The hormone levels were measured monthly. When E2 and FSH levels were stable at postmenopausal standard, E2 and FSH levels were tested once every 3-6 months.
Clinicopathologic factors were used to evaluate recurrence and death due to breast cancer. These factors included age, pT stage, pN stage, molecular subtypes, neoadjuvant chemotherapy, and ovarian ablation (goserelin treatment). Patients were defined as ER and/or PR positive when immunohistochemistry showed that the proportion of ER and/or PR positive cells was ≥ 10%; patients were defined as positive for Her-2 when immunohistochemistry for Her-2 showed 3+ or 2+ with confirmation by fluorescence in situ hybridization. In this study, immunohistochemistry for Ki-67 was not performed in many patients, thus molecular subtypes were not determined according to the St. Gallen International Expert Consensus on Primary Therapy of Early Breast Cancer 2011 [12]. Therefore, traditional molecular subtypes were classified, including luminal A (ER and/or PR positive, Her-2 negative) and luminal B (ER and/or PR positive, Her-2 positive).
After comprehensive therapy, patients received regular follow-up. For detection of local or distant relapse, patients were scheduled for clinical follow-up every six to 12 months, including recording patient’s history, physical examination, laboratory tests of complete blood counts, liver function test, chest radiography, mammography, breast and abdominopelvic ultrasonography, and bone scans. In addition, a computed tomography scan or a fluorine-18-fluorodeoxyglucose positron emission tomography scan was performed if necessary. Locoregional recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS) were the primary endpoints. Locoregional recurrence referred to pathologically confirmed recurrence at the ipsilateral chest wall, or within supraclavicular and subclavian lymph nodes, axillary lymph nodes, or internal mammary lymph nodes. Distant metastasis referred to recurrence at a site distal to the primary cancer, confirmed by two imaging examinations or pathologically confirmed (and pathology assessment, if needed). DFS referred to absence of locoregional or distant recurrence. Death was defined as death due to breast cancer.
All data were analyzed using the SPSS ver. 16.0 (SPSS Inc., Chicago, IL). The Kaplan-Meier method was used for generation of survival plots and for comparison of survival rates. Statistical significance was determined using the Log-rank test. Stepwise Cox regression analysis was used for multivariate analysis. Factors that were significant indicators of endpoints in univariate analysis were included in the stepwise Cox regression analysis. p-values below 0.05 were considered significant.
Between October 1999 and November 2007, 1,332 patients were diagnosed as stage II/III hormone receptor-positive breast cancer and were treated according to international guidelines; 840 patients (63.1%) were premenopausal and 353 of them were without CIA, 492 patients were postmenopausal. As shown in Table 1, a total of 353 patients with a median age (at disease onset) of 41 years (range, 24 to 50 years) were recruited. Of these patients, 69 (19.5%) received neoadjuvant chemotherapy with regimens containing anthracycline and/or taxanes and the median number of cycles of chemotherapy was 2 (range, 2 to 6 cycles). All patients received adjuvant chemotherapy, and 343 patients received regimens with anthracycline and/or taxanes and 10 patients received regimens containing cyclophosphamide, methotrexate, and 5-fluorouracil (CMF). The median number of cycles of adjuvant chemotherapy was 4 (range, 2 to 6 cycles).
For patients without neoadjuvant chemotherapy, the median number of cycles of adjuvant chemotherapy was 6 (range, 4 to 8 cycles). Adjuvant radiotherapy was mainly delivered to the ipsilateral chest wall, supraclavicular and infraclavicular region after mastectomy. The total radiation dose was 50 Gy with 2 Gy delivered over 25 times. However, no patient received herceptin.
After adjuvant radiotherapy, endocrine therapy was initiated. Of these patients, 255 patients (72.2%) were treated with tamoxifen (TAM) without ovarian suppression; 98 (27.8%) were treated with TAM and goserelin once monthly for a median duration of 31 months (range, 24 to 59 months), 83/98 patients (84.7%) were treated based on resumption or persistence of menstruation, and 15/98 patients (15.3%) were treated based on hormone levels. After completion of goserelin treatment, 68 patients (69.4%) recovered their menstrual cycle within one year and the remaining 30 patients developed amenorrhea (30.6%).
The median follow-up period was 61 months (range, 12 to 144 months). Locoregional recurrence was observed in 16 patients. The 5- and 10-year LRFS were 95.8% and 94.1%, respectively. Distant metastasis was noted in 90 patients, and the 5- and 10-year DMFS were 75.6% and 62.3%, respectively. The 5- and 10-year DFS were 75.4% and 59.4%, respectively. In addition, 61 patients died of breast cancer, and the 5- and 10-year OS were 86.1% and 63.1%, respectively.
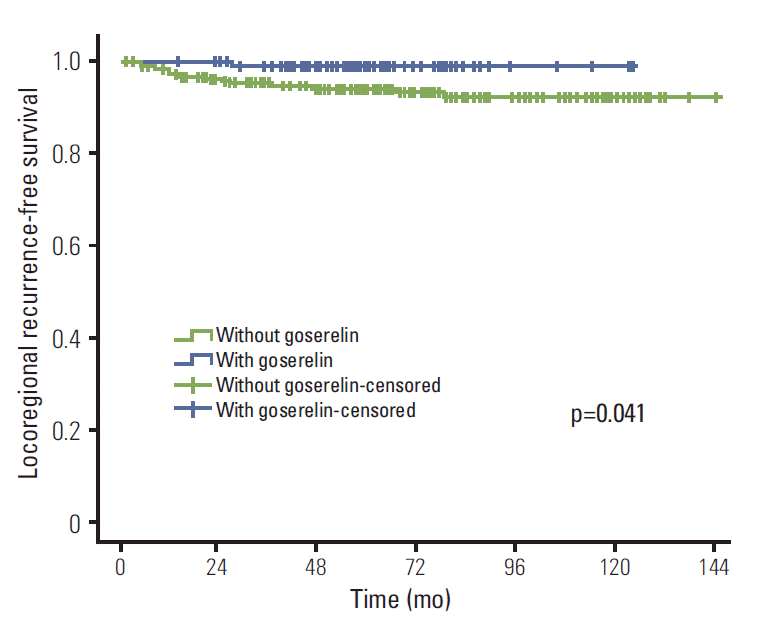
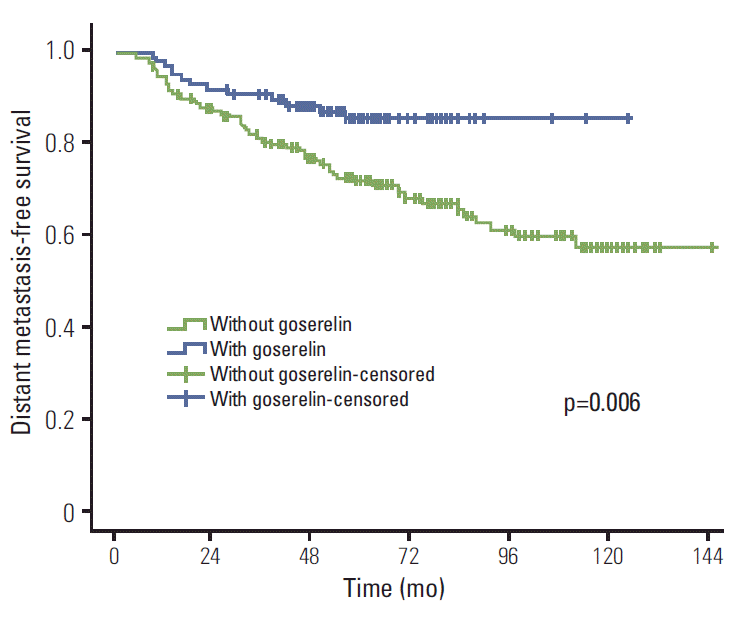
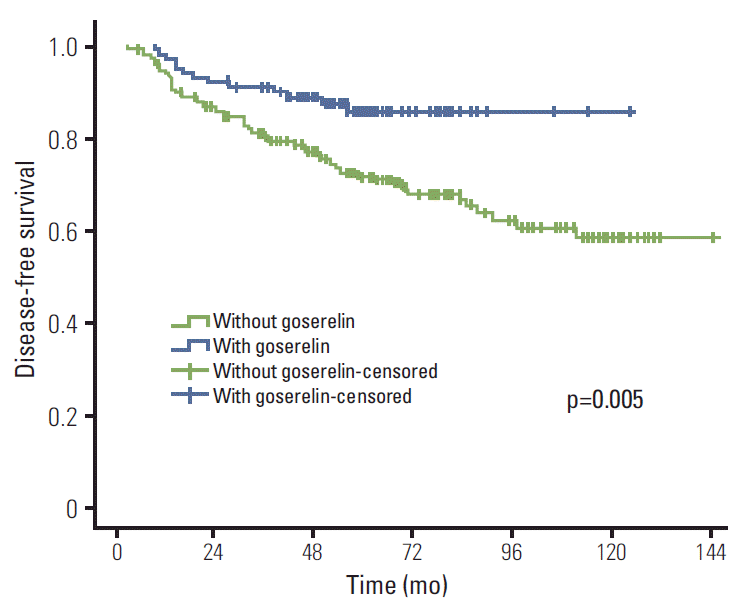
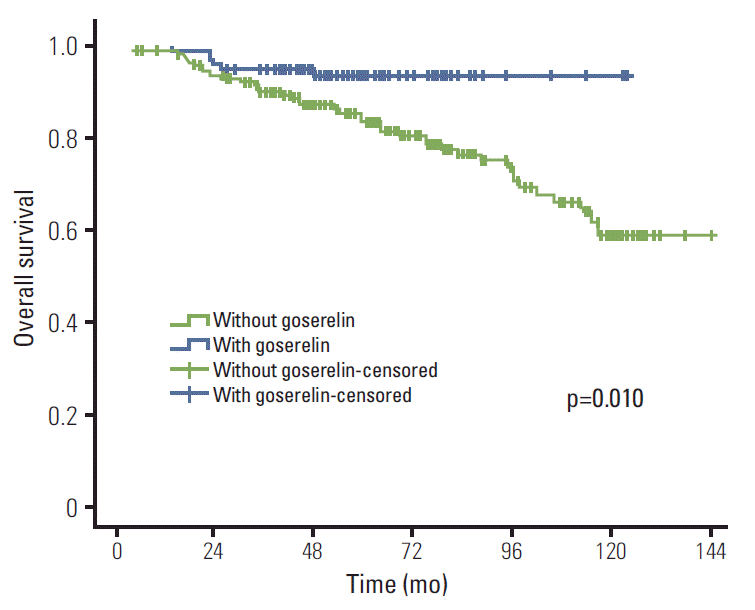
Results of univariate analysis are shown in Table 2. Treatment with goserelin (when compared with goserelinuntreated patients) resulted in significantly improved LRFS (98.9% vs. 94.1%, p=0.041), DMFS (85.4% vs. 71.9%, p=0.006), DFS (85.4% vs. 71.6%, p=0.005), and OS (93.5% vs. 83.5%, p=0.010) (Figs. 1-4). In addition, molecular subtypes, pN stage, and neoadjuvant chemotherapy also influenced breast cancer prognosis (p < 0.05).
In multivariate analysis, goserelin treatment was an independent factor influencing DMFS (hazard ratio [HR], 1.603; 95% confidence interval [CI], 1.228 to 2.092; p=0.001), DFS (HR, 1.606; 95% CI, 1.231 to 2.096; p=0.001), and OS (HR, 3.311; 95% CI, 1.416 to 7.742; p=0.006) (Table 3). Traditional clinicopathological factors, including pN stage and neoadjuvant chemotherapy, also had independent prognostic power in the Cox’s models. A statistically significant relationship with poor survival was observed for patients with increasing pN stage and neoadjuvant chemotherapy (p < 0.05).
The influence of age on the therapeutic efficacy of goserelin is shown in Table 4. Treatment with goserelin resulted in significantly improved LRFS (p=0.039), DMFS (p=0.043), DFS (p=0.036), and OS (p=0.010) in patients aged < 40 years. In patients aged ≥ 40 years, treatment with goserelin resulted in significantly improved DMFS (p=0.028) and DFS (p=0.027), but had no influence on LRFS (p=0.501) or OS (p=0.210).
This study assessed the value of goserelin in treatment of premenopausal patients with stage II/III hormone receptorpositive breast cancer without CIA. According to our results, ovarian ablation (goserelin treatment), in combination with TAM, significantly improved the LRFS, DMFS, DFS, and OS of these patients.
Ovarian ablation is achieved by inhibiting the secretion of luteinizing hormone and FSH in the anterior pituitary, which subsequently leads to inhibition of estrogen secretion in the ovary. Theoretically, this therapy may reduce the risk of breast cancer recurrence and death in premenopausal hormone receptor-positive patients. However, the role of goserelin as adjuvant therapy for breast cancer is still controversial. Kaufmann et al. [13] reported that goserelin did not improve survival in breast cancer patients undergoing adjuvant chemotherapy. Sverrisdottir et al. [14] also found that the therapeutic efficacy of TAM in combination with goserelin was not superior to that of TAM alone. However, Cheng et al. [15] reported that breast cancer patients treated with goserelin had significantly prolonged survival compared to patients receiving adjuvant chemotherapy (11-year OS, 88% vs. 82%; p=0.002). Findings of the International Breast Cancer Study Group Trial VIII (IBCSG-VIII) showed that endocrine therapy that included goserelin improved DFS of breast cancer patients [16]. Findings of the study of Zoladex in Pre-menopausal Patients (ZIPP) also showed that treatment with goserelin improved their eventfree survival and OS [17]. A meta-analysis of LHRH-agonists in the Early Breast Cancer Overview group showed that treatment with goserelin alone had efficacy similar to that of adjuvant chemotherapy, and that addition of LHRH-agonists (in the presence of adjuvant chemotherapy and TAM) further reduced the risk of recurrence (p=0.02) and death (p=0.03) [6]. Based on the above findings and our results, we speculate that ovarian ablation with goserelin in combination with TAM may improve the survival of premenopausal breast cancer patients who are hormone receptor-positive.
The inclusion criteria, the patterns of adjuvant therapy, and the menstrual status before goserelin therapy may influence therapeutic efficacy. In previous studies, most patients were premenopausal, and, thus, the inclusion of patients was less dependent on the menstrual cycle and serum hormone levels. However, studies have shown that patients with CIA usually have a better prognosis, which is consistent with the suppression of ovarian function [18]. In the current study, all patients had regular menstrual cycles or hormone levels similar to premenopausal levels, suggesting that these patients had active ovarian function after adjuvant chemotherapy, which may have influenced the prognosis. Findings of the IBCSG study also indicate that LHRHagonists can improve the therapeutic efficacy of patients without CIA [19], which is consistent with our findings.
Anthracyclines and alkylating agents commonly used in chemotherapy of breast cancer were reported to induce CIA in 0-46% of patients aged < 40 years and in 65-100% of patients aged ≥ 40 years [6,16,20,21]. Findings of the IBCSGVIII study and International Adjuvant Breast Cancer Ovarian Ablation or Suppression Randomized Trial also indicated that patients under 40 years of age would benefit to a greater extent from goserelin treatment when compared with those ≥ 40 years of age. However, in the two studies mentioned above, only the menstrual cycle at disease onset was considered, and CIA was not taken into account [11,16]. In the current study, patients without CIA who received goserelin had therapeutic efficacy seen in those aged < 40 years and those aged ≥ 40 years (Table 4). According to the guidelines, both the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) for treatment of breast cancer recommend that ovarian ablation serve as a pattern of adjuvant therapy in premenopausal patients, however, the optimal population for this therapy has not yet been determined [11,22]. According to our findings, we postulate that patients without CIA before menopause are suitable for ovarian ablation. Two studies to clarify the role of ovarian suppression in combination with hormonal treatment in premenopausal women (Suppression of Ovarian Function Trial [SOFT] and Tamoxifen and Exemestane Trial [TEXT]) are ongoing [23].
Our study had several limitations. This retrospective study was conducted without randomization in a small patient population at a single medical center. Thus, our results should be confirmed using a prospective, randomized, controlled trial including a larger patient population. The study enrolled patients treated with goserelin referring to ESMO’s recommendation for at least two years [11]. In ongoing studies, this treatment lasts for 3-5 years in most patients [23]. In the current study, herceptin was not administered in these patients, and whether or not targeted therapy might improve outcome is still unclear.
ACKNOWLEDGMENTS
This study was supported by a grant from the Sci-Tech Office of Guangdong Province (No. 2008B060600019), the Youth Foundation of the First Affiliated Hospital of Xiamen University (No. XYY2012005), and the Education Scientific Research Project of Young Teachers in Fujian Province (No. JB13131).
References
1. Li J, Zhang BN, Fan JH, Pang Y, Zhang P, Wang SL, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011; 11:364.

2. Han JG, Jiang YD, Zhang CH, Pang D, Zhang M, Wang YB, et al. Clinicopathologic characteristics and prognosis of young patients with breast cancer. Breast. 2011; 20:370–2.

3. Peng R, Wang S, Shi Y, Liu D, Teng X, Qin T, et al. Patients 35 years old or younger with operable breast cancer are more at risk for relapse and survival: a retrospective matched case-control study. Breast. 2011; 20:568–73.

4. Tan SH, Wolff AC. Luteinizing hormone-releasing hormone agonists in premenopausal hormone receptor-positive breast cancer. Clin Breast Cancer. 2007; 7:455–64.

5. Clarke MJ. Ovarian ablation in breast cancer, 1896 to 1998: milestones along hierarchy of evidence from case report to Cochrane review. BMJ. 1998; 317:1246–8.
6. LHRH-agonists in Early Breast Cancer Overview Group, Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007; 369:1711–23.
7. Wu S, Li Q, Zhu Y, Sun J, Li F, Lin H, et al. Role of goserelin in combination with endocrine therapy for the treatment of advanced breast cancer in premenopausal women positive for hormone receptor: a retrospective matched case-control study. Cancer Biother Radiopharm. 2013; 28:697–702.

8. Davidson NE, O'Neill AM, Vukov AM, Osborne CK, Martino S, White DR, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101(E5188). J Clin Oncol. 2005; 23:5973–82.
9. Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009; 360:679–91.

10. Adjuvant Breast Cancer Trials Collaborative Group. Ovarian ablation or suppression in premenopausal early breast cancer: results from the international adjuvant breast cancer ovarian ablation or suppression randomized trial. J Natl Cancer Inst. 2007; 99:516–25.
11. Aebi S, Davidson T, Gruber G, Cardoso F; ESMO Guidelines Working Group. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011; 22 Suppl 6:vi12–24.

12. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.
13. Kaufmann M, Graf E, Jonat W, Eiermann W, Vescia S, Geberth M, et al. A randomised trial of goserelin versus control after adjuvant, risk-adapted chemotherapy in premenopausal patients with primary breast cancer - GABG-IV B-93. Eur J Cancer. 2007; 43:2351–8.

14. Sverrisdottir A, Johansson H, Johansson U, Bergh J, Rotstein S, Rutqvist L, et al. Interaction between goserelin and tamoxifen in a prospective randomised clinical trial of adjuvant endocrine therapy in premenopausal breast cancer. Breast Cancer Res Treat. 2011; 128:755–63.

15. Cheng TF, Wang JD, Uen WC. Cost-utility analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy in premenopausal women with breast cancer. BMC Cancer. 2012; 12:33.

16. Karlsson P, Sun Z, Braun D, Price KN, Castiglione-Gertsch M, Rabaglio M, et al. Long-term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer. Ann Oncol. 2011; 22:2216–26.

17. Baum M, Hackshaw A, Houghton J, Rutqvist , Fornander T, Nordenskjold B, et al. Adjuvant goserelin in pre-menopausal patients with early breast cancer: results from the ZIPP study. Eur J Cancer. 2006; 42:895–904.

18. Pagani O, O'Neill A, Castiglione M, Gelber RD, Goldhirsch A, Rudenstam CM, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998; 34:632–40.

19. Goldhirsch A, Gelber RD, Yothers G, Gray RJ, Green S, Bryant J, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001; 30:44–51.

20. Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006; 24:5769–79.

21. Arriagada R, Le MG, Spielmann M, Mauriac L, Bonneterre J, Namer M, et al. Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. Ann Oncol. 2005; 16:389–96.

22. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, Breast Cancer. Version 1, 2011 [Internet]. Fort Washington: National Comprehensive Cancer Network;[cited 2010 Nov 8]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
Table 1.
Clinicopathologic characteristics of patient groups
Table 2.
Univariate analysis of factors influencing prognosis of breast cancer patients
| Characteristic |
LRFS |
DMFS |
DFS |
OS |
||||
|---|---|---|---|---|---|---|---|---|
| 5-year (%) | p-value | 5-year (%) | p-value | 5-year (%) | p-value | 5-year (%) | p-value | |
| Age (yr) | ||||||||
| < 40 | 95.2 | 0.514 | 72.5 | 0.054 | 72.2 | 0.074 | 83.5 | 0.314 |
| ≥ 40 | 96.3 | 78.3 | 77.8 | 87.9 | ||||
| pT stage | ||||||||
| pT1 | 93.5 | 0.770 | 68.3 | 0.101 | 66.0 | 0.104 | 89.3 | 0.394 |
| pT2 | 96.3 | 80.6 | 80.7 | 88.7 | ||||
| pT3 | 97.0 | 68.7 | 68.7 | 79.8 | ||||
| pT4 | 93.2 | 68.3 | 68.3 | 76.8 | ||||
| pN stage | ||||||||
| pN0 | 92.9 | 0.195 | 85.7 | 0.005a) | 85.7 | 0.005a) | 83.9 | 0.010a) |
| pN1 | 98.3 | 88.5 | 88.6 | 97.1 | ||||
| pN2 | 97.0 | 77.0 | 77.0 | 87.6 | ||||
| pN3 | 93.3 | 65.5 | 64.7 | 78.1 | ||||
| Molecular subtypes | ||||||||
| Luminal-A | 96.9 | 0.041a) | 76.7 | 0.321 | 76.8 | 0.302 | 87.7 | 0.668 |
| Luminal-B | 93.4 | 72.9 | 72.0 | 82.6 | ||||
| Neoadjuvant chemotherapy | ||||||||
| No | 96.6 | 0.038a) | 78.5 | 0.007a) | 78.2 | 0.008a) | 88.1 | 0.130 |
| Yes | 92.4 | 63.4 | 63.5 | 78.1 | ||||
| Goserelin | ||||||||
| No | 94.1 | 0.041a) | 71.9 | 0.006a) | 71.6 | 0.005a) | 83.5 | 0.010a) |
| Yes | 98.9 | 85.4 | 85.4 | 93.5 | ||||
Table 3.
Multivariate analysis of factors influencing prognosis of breast cancer patients
| Characteristic |
LRFS |
DMFS |
DFS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% Cl | p-value | HR | 95% Cl | p-value | HR | 95% Cl | p-value | HR | 95% Cl | p-value | |
| pN stage | - | - | - | 1.603 | 1.228-2.092 | 0.001a) | 1.606 | 1.231-2.096 | < 0.001a) | 1.745 | 1.253-2.431 | 0.006a) |
| Molecular subtypes | 2.282 | 0.880-5.923 | 0.090 | - | - | - | - | - | - | - | - | - |
| Neoadjuvant chemotherapy | 2.973 | 1.092-8.092 | 0.033a) | 1.970 | 1.229-3.159 | 0.005a) | 1.968 | 1.227-3.156 | 0.005a) | - | - | - |
| Without goserelin | 7.262 | 0.954-55.298 | 0.056 | 1.603 | 1.228-2.092 | 0.001a) | 1.606 | 1.231-2.096 | 0.001a) | 3.311 | 1.416-7.742 | 0.006a) |
Table 4.
Influence of age on therapeutic efficacy of goserelin
| Survival endpoint | Without goserelin | With goserelin | p-value |
|---|---|---|---|
| < 40 years | |||
| 5-Year LRFS (%) | 92.7 | 100 | 0.039a) |
| 5-Year DMFS (%) | 68.1 | 81.5 | 0.043a) |
| 5-Year DFS (%) | 67.2 | 81.5 | 0.036a) |
| 5-Year OS (%) | 78.0 | 94.1 | 0.010a) |
| ≥ 40 years | |||
| 5-Year LRFS (%) | 95.1 | 97.6 | 0.501 |
| 5-Year DMFS (%) | 74.2 | 89.7 | 0.028a) |
| 5-Year DFS (%) | 74.3 | 89.7 | 0.027a) |
| 5-Year OS (%) | 86.2 | 92.7 | 0.210 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download