Abstract
Purpose
The purpose of this study is to retrospectively compare the efficacy and tolerability between three regimens for first-line chemotherapy—gemcitabine plus capecitabine (GEM-X), gemcitabine plus erlotinib (GEM-T), and gemcitabine monotherapy (GEM)—in patients with advanced pancreatic cancer.
Materials and Methods
There was a total of 127 patients who underwent chemotherapy for pancreatic cancer between January 2007 and November 2011 at our institution. Patients were treated with either GEM (gemcitabine 1,000 mg/m2 on days 1, 8, and 15 every 4 weeks), GEM-T (gemcitabine 1,000 mg/m2 on days 1 and 8 every 3 weeks and erlotinib 100 mg daily), or GEM-X (gemcitabine 1,000 mg/m2 on days 1 and 8 every 3 weeks and capecitabine 850 mg/m2 twice daily for 2 weeks followed by 1 week’s rest) as the first-line treatment. Progression-free survival (PFS), overall survival (OS), objective response rate (ORR), and toxicity were evaluated.
Results
The patient population was divided into groups depending on their first-line treatment: GEM (n=47), GEM-T (n=44), and GEM-X (n=36). GEM-X significantly improved ORR (21.2% vs. 12.7% and 15.9%), PFS (8.9 vs. 5.2 and 3.9 months; p < 0.001), and OS (12.1 vs. 10.4 and 9.9 months; p = 0.03) compared to GEM and GEM-T, respectively. There were higher incidences of some non-hematologic adverse events with GEM-X and GEM-T compared to GEM, but most were grade 1 or 2.
Conclusion
GEM-X presented better clinical efficacy and acceptable tolerability than GEM-T and GEM in advanced pancreatic cancers. It is worthy to further investigate which agent has a clinical advantage as a combination drug with gemcitabine in pancreatic cancer and to explore the predictive markers leading to personalize anti-cancer treatment.
Pancreatic cancer is one of the most desperate cancers, causing approximately 266,000 deaths worldwide in 2008 [1]. The majority of these cases are diagnosed at unresectable stages, and the prognosis is extremely poor, despite curative resection. Currently, the median overall survival of patients with locally advanced and metastatic pancreatic cancers is only 6 to 12 months if treated with standard chemotherapy [2-5].
Fifteen years ago, gemcitabine was used as a first-line chemotherapy to extend the overall survival (OS) to 5.65 months from 4.41 months in advanced pancreas cancer patients who received fluorouracil [2]. Therefore, gemcitabine became the standard treatment for patients in advanced stages. Since then, randomized phase III trials of cytotoxic or biologic drugs (erlotinib [3], capecitabine [4,5], fluorouracil [6], oxaliplatin [7], cisplatin [8]), combined with gemcitabine, have exhibited potential efficacy compared with gemcitabine alone [9].
Of the phase III clinical trials, erlotinib (Tarceva; Roche) have presented promising results as a gemcitabine combination partner in pancreatic cancer. Erlotinib combined with gemcitabine demonstrated an improvement in survival compared to gemcitabine monotherapy (GEM; median, 6.24 months vs. 5.91 months; p=0.038) without improving the objective response rate (ORR) [3]. Currently, GEM and gemcitabine plus erlotinib (GEM-T) combination have been accepted as first-line chemotherapies for metastatic pancreatic cancer based on high-level evidence [9,10]. Though capecitabine combined with gemcitabine improved ORR (19.1% vs. 12.4%, p=0.034) and progression-free survival (PFS) (hazard ratio [HR], 0.78; p=0.004) of pancreatic cancer patients, survival benefit of the regimen was not significant (HR, 0.86; p=0.08) compared with gemcitabine alone [4]. However, several meta-analysis reported that gemcitabine combined with capecitabine presented a significant increase in survival compared to gemcitabine alone [11,12].
The issue of which agent may be the best combination with gemcitabine for pancreas cancer has not been studied thus far. The aim of this study is to retrospectively compare the efficacy and toxicity of capecitabine or erlotinib in combination with gemcitabine compared with gemcitabine alone in patients with locally advanced or metastatic pancreatic cancer, and to determine which regimen exhibits a more clinical benefit as a first line chemotherapy in pancreatic cancer.
We collected the data of patients who were diagnosed as inoperable pancreatic adenocarcinoma between January 2007 and November 2011 at the Gangnam Severance Cancer Hospital in South Korea. Patients enrollment eligibility was as follows: histologically or cytologically confirmed ductal adenocarcinoma of the pancreas; unresectable/metastatic disease; treated with one of GEM, gemcitabine plus capecitabine (GEM-X), and GEM-T as first-line treatment; measurable or evaluable lesion; age more than 18 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0, 1, or 2; and adequate hematologic (granulocyte count, ≥ 1,500×106/L; platelet count, ≥100×109/L), hepatic (bilirubin < 1.5× upper limit of normal [ULN]), and renal function (serum creatinine < 1.5× ULN) before first-line chemotherapy. Prior radiotherapy for local disease was allowed if the disease progression had been documented and if radiotherapy was completed at least 4 weeks before enrollment. Prior chemotherapy was not permitted, except for postoperative adjuvant treatment or radiosensitizers.
Chemotherapy regimen for each patient was determined by the physician in charge. For the GEM group, gemcitabine (1,000 mg/m2) was given by a 30-minute intravenous infusion on days 1, 8 and 15, every 4 weeks. For GEM-X and GEM-T groups, gemcitabine (1,000 mg/m2) was administered on days 1 and 8, in 3-week cycles. Erlotinib was taken orally at 100 mg daily. Capecitabine was administered orally at 1,700 mg/m2/day (850 mg/m2 twice daily) for 2 weeks, followed by a 1-week rest. All treatments were administered until disease progression or intolerable toxicity. The chemotherapy doses could be reduced or delayed (no more than 1 cycle) to allow recovery from toxicity. Dose reduction in gemcitabine and capecitabine was in accordance to two levels, 850 mg/m2 (level 1) and 700 mg/m2 (level 2) for gemcitabine and 1,500 mg/m2 (level 1) and 1,200 mg/m2 (level 2) for capecitabine. Patients with poorly tolerated diarrhea, skin adverse drug reaction, or any other adverse events related with erlotinib, were managed with drug interruption, followed by a restart of erlotinib. This study was approved by the Institutional Review Board of the Yonsei University.
The pretreatment baseline evaluation included a complete medical history, a physical examination, vital signs, electrocardiogram, chest radiography, and routine laboratory tests. The serum carbohydrate antigen (CA) 19-9 level was assessed at the baseline. Tumor assessment was performed by a spiral computed tomography scan at the baseline, every 2 cycles. The radiologic tumor response was evaluated in accordance to the Response Evaluation Criteria in Solid Tumors (RECIST). Toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria ver. 3.0. Clinical and laboratory assessments were conducted throughout the study.
The primary end point was PFS, and the secondary end points were OS and ORR. PFS was calculated from the date of initial drug administration to the date of either disease progression or death, or it was censored at the last follow up. OS was calculated from the date of initial drug administration to the date of death from any cause, or it was censored at the last follow up. Both PFS and OS estimates were calculated using the Kaplan-Meier method, and the survival curves were compared among the treatment arms using the log-rank test. The HR with 95% confidence interval (CI) was used as the primary estimate of the difference between the two arms. The effects of potential prognostic factors were assessed using the Cox’s proportional hazards model. The proportions were compared across the groups using the χ2 test or Fisher’s exact test. Statistical analyses were done with PASW statistics ver. 18.0 (SPSS Inc., Chicago, IL). A p < 0.05 was considered to be statistically significant.
A total of 127 patients were enrolled in this study. Patients received one of the following treatments: GEM (n=47), GEM-T (n=44), or GEM-X (n=36). The median follow-up duration was 27.8 months (range, 1.5 to 71.9 months). Their baseline characteristics are described in Table 1. Patients in the GEM arm were statistically older than the others. Prognostic factors, such as ECOG PS, disease extent, and baseline CA 19-9 level, were not different between the three groups, except disease extent between the GEM and GEM-T arms. The proportion of locally advanced disease, which presents a better prognosis than distant and recurred diseases, was higher in the GEM arm than in the GEM-T arm (44.7 vs. 25%, p=0.049). Locally advanced disease was present in 36.2% of all cases. The pancreatic head was the most common location of the primary lesions (57.5%). The CA 19-9 level was over the upper normal limit in 107 patients (84.3%). Patients were grouped into high or low CA 19-9 group by the median level of 500 U/mL. Prior chemotherapy and radiotherapy were administered as adjuvant treatments after curative resection of pancreatic cancer.
After the protocol-specified treatment, 69 patients received further treatments. A second-line chemotherapy was administered to 31.9%, 52.3%, and 27.7% of patients in the GEM, GEM-T, and GEM-X arms, respectively. The selection of a second-line chemotherapy was up to the individual investigator, and 5-fluorouracil or platinum-based regimens were mostly used.
One hundred twenty-four patients were assessed for response. One patient was lost to follow-up right after the first dose of chemotherapy, and two patients did not undergo the first follow-up tumor assessment due to their refusal. Table 2 presents a summary of the response results. Patients in the GEM-X arm had an improved partial response rate over GEM and GEM-T (21.2% vs. 12.7% and 15.9%, respectively) and improved the overall disease control rate (partial response plus stable disease; 72.7% vs. 63.8% and 59.1%, respectively); however, there was no statistical significance. The treatment duration was significantly extended in the GEM-X group. The median duration of gemcitabine treatment was 17, 10.4, and 22.8 weeks in the GEM, GEM-T, and GEM-X groups, respectively.
PFS was significantly prolonged in the GEM-X group compared to the GEM and GEM-T groups (GEM-X vs. GEM; median, 8.9 vs. 5.2 months; HR, 0.46; 95% CI, 0.27 to 0.79; p=0.035, and GEM-X vs. GEM-T; median, 8.9 vs. 3.9 months; HR, 0.36; 95% CI, 0.2 to 0.64; p=0.001). GEM-X significantly reduced the hazard of progression compared with GEM and GEM-T (Fig. 1A). The OS was also significantly improved with GEM-X compared to GEM (median OS, 12.1 vs. 10.4 months; HR, 0.52; 95% CI, 0.28 to 0.94; p=0.033) or to GEM-T (median OS, 12.1 vs. 9.9 months; HR, 0.52; 95% CI, 0.28 to 0.96; p=0.037) (Fig. 1B). The 1-year survival rates were 44.4% for GEM-X, 25.5% for GEM, and 22.7% for GEM-T. In the three arms, both partial response and stable disease extended PFS and OS compared to the progressive disease. However, there was no significant difference in clinical benefit between partial response and stable disease (all p > 0.05 in PFS and OS).
In the analysis of GEM-X compared to GEM-T, the results of the subgroup analyses of survival by the baseline characteristic and clinical factor are displayed in Table 3. GEM-X significantly reduced the hazard of progression compared with GEM-T in all cases, except high CA 19-9 patients. A statistically significant benefit of GEM-X in OS was confined to patients with age less than 65.
All 127 patients received at least one dose of medication and were available for an assessment of its toxicity. The median number of cycles was 4 (range, 1 to 14), 4 (range, 1 to 21), and 7 (range, 1 to 20) cycles in the GEM, GEM-T, and GEM-X groups, respectively. A total of 239 cycles of GEM, 236 cycles of GEM-T, and 272 cycles of GEM-X were administered. Adverse events are summarized in Table 4. The treatments were generally well tolerated in all arms. The relative dose intensity of gemcitabine was 93% for GEM, 98% for GEM-T, and 94% for GEM-X. The relative dose intensity of erlotinib was 96% and of capecitabine was 78%. Patients in the GEM-T arm experienced higher frequencies of rash and diarrhea, but these were generally grade 1 or 2. The GEM-X arm was related to more frequent neutropenia and grade 1 or 2 hand-foot syndrome. GEM arm showed more anemia and thrombocytopenia. Infection was more common in the GEM arm, and the main cause of infection was biliary obstruction (80%). Due to older age and ECOG PS 2 patients having received GEM, it is suggested that anemia, thrombocytopenia, and infection may be related more with poor patients’ baseline condition rather than chemotherapeutic regimen. Of the patients receiving GEM or GEM-T, 27% had underwent at least one gemcitabine dose reduction. The main cause of dose modification was hematologic toxicity. A higher proportion (33%) of patients had a dose reduction in the GEM-X arm than in other arms. The dose of capecitabine was reduced in 25% of GEM-X patients (Table 4).
We investigated the comparative efficacy of three regimens, GEM, GEM-T, and GEM-X in pancreatic cancer. GEM-X was superior to GEM and GEM-T, and GEM-T presented a similar efficacy to GEM. The OS was improved with GEM-X compared to GEM or to GEM-T. GEM-X also improved the RR and PFS compared to GEM and GEM-T. In all groups, metastatic disease, high CA 19-9, and ECOG 2 were related with poor prognosis.
In a comparative analysis of GEM-X and GEM-T, there was no difference in the extent of disease, age, and ECOG PS of patients. The improvement in PFS with HR of 0.36 supports the beneficial effects of capecitabine over erlotinib. The reduced hazard of progression by capecitabine was consistent across the different subgroups (Table 3). To a lesser extent, the OS benefit of GEM-X was also consistent across all subgroups, especially in patients who were less than the age 65 years, despite age being not a significant prognostic factor of PFS or OS in the total analysis. A recent retrospective study supports our results. Compared with GEM-T, GEM-X exhibited a similar response rate (23.5% vs. 21.1%), but had a better disease control rate (52.9% vs. 73.7 %) along with a presentation of longer PFS (2.63 vs. 5.37 months, p=0.032) and OS (6.23 vs. 14.43 months, p=0.002) [13].
The superior efficacy of GEM-X was accompanied by acceptable levels of toxicity. GEM-X was well tolerated and not hard to administer compared to GEM or GEM-T. The relative dose intensity of gemcitabine was not different between the two groups; 98% for GEM-T and 94% for GEM-X, and erlotinib was 96% and of capecitabine was 78%. The dose of capecitabine was reduced for 25% of patients in the GEM-X arm, mostly owing to grade 3 or 4 neutropenia without serious events. There was no other frequent grade 3 or 4 adverse event in the GEM-X arm.
Although erlotinib plus gemcitabine has presented superior survival over gemcitabine alone in a phase III trial, the median survival gain was only 0.33 months [3]. GEM-X has shown clinical benefit in phase II and III trials for pancreatic cancer [4,5,14]. Our findings indicate that it would be of great value to further explore the clinical advantage of GEM-X over other gemcitabine combination regimens for unresectable pancreatic cancer. GEM-T presented a rather disappointing clinical efficacy in our study. This result indicates that capecitabine may have a more potent antitumor effect than erlotinib in pancreatic cancer. According to the national treatment guideline, capecitabine monotherapy is an option for patients with poor PS or second-line chemotherapy, but not erlotinib. To select patients who would benefit from erlotinib, it is mandatory to identify the potential predictive markers. In non-small cell lung cancer, the diversity of response to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor has been recognized through several clinical trials [15,16]. EGFR mutation testing was established as a mandatory process for using erlotinib as a first-line treatment in non-small cell lung cancer [17,18]. Additionally, application of potential predictive markers (thymidylate synthase and thymidine phosphorylase) for capecitabine might lead to select patients in practice [19,20].
Though FOLFIRINOX consisting of oxaliplatin, irinotecan, fluorouracil, and leucovorin was considered as one of the first-line regimens for pancreatic cancer [21], gemcitabine based combination regimens are currently considered as the standard treatment. It is necessary to find the best combination for each patient based on predictive biomarker or patient’s characteristics. Several potential limitations in our study need to be considered. This study is a non-prospective and non-randomized investigation with relatively a small patient population. Our study may provide evidence for revisiting the clinical benefit of GEM-X regimen, and validation studies with large and randomized phase III trials are required.
This study was performed to evaluate the efficacy of capecitabine in combination with gemcitabine compared with gemcitabine with or without erlotinib in patients with locally advanced or metastatic pancreatic cancer. The addition of capecitabine to gemcitabine presented promising outcomes over GEM-T in pancreatic cancer. It is worthy to further investigate which agent has the clinical advantage as a combination drug with gemcitabine for pancreatic cancer and to explore predictive markers for each regimen leading to personalized anti-cancer treatment.
ACKNOWLEDGMENTS
This study was supported by faculty research grant from Yonsei University College of Medicine for 2011 (6-2011-0113, 6-2011-0146).
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

2. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15:2403–13.

3. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25:1960–6.

4. Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009; 27:5513–8.

5. Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007; 25:2212–7.

6. Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002; 20:3270–5.

7. Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005; 23:3509–16.

8. Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006; 24:3946–52.

9. Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010; 7:163–72.

10. Oberstein PE, Saif MW. First-line treatment for advanced pancreatic cancer. Highlights from the "2011 ASCO Gastrointestinal Cancers Symposium". San Francisco, CA, USA. January 20-22, 2011. JOP. 2011; 12:96–100.
11. Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008; 8:82.

12. Sultana A, Tudur Smith C, Cunningham D, Starling N, Tait D, Neoptolemos JP, et al. Systematic review, including metaanalyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer. 2007; 96:1183–90.

13. Jeon EK, Won HS, Ko YH, Lee IS, Hong TH, You YK, et al. Comparison of the efficacy and the toxicity between gemcitabine with capecitabine (GC) and gemcitabine with erlotinib (GE) in unresectable pancreatic cancer. J Cancer Res Clin Oncol. 2012; 138:1625–30.

14. Choi JG, Seo JH, Oh SC, Choi CW, Kim JS. A phase II trial of gemcitabine plus capecitabine for patients with advanced pancreatic cancer. Cancer Res Treat. 2012; 44:127–32.

15. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005; 366:1527–37.

16. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.

17. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.

18. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–8.

19. Lee SJ, Choi YL, Park YH, Kim ST, Cho EY, Ahn JS, et al. Thymidylate synthase and thymidine phosphorylase as predictive markers of capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Cancer Chemother Pharmacol. 2011; 68:743–51.

Fig. 1.
Kaplan-Meier curves for PFS (A) and OS (B) of the GEM (red line), GEM-T (green line), and GEM-X arms (blue line). PFS, progression-free survival; OS, overall survival; GEM, gemcitabine; GEM-T, gemcitabine plus erlotinib; GEM-X, gemcitabine plus capecitabine; HR, hazard ratio; CI, confidence interval.
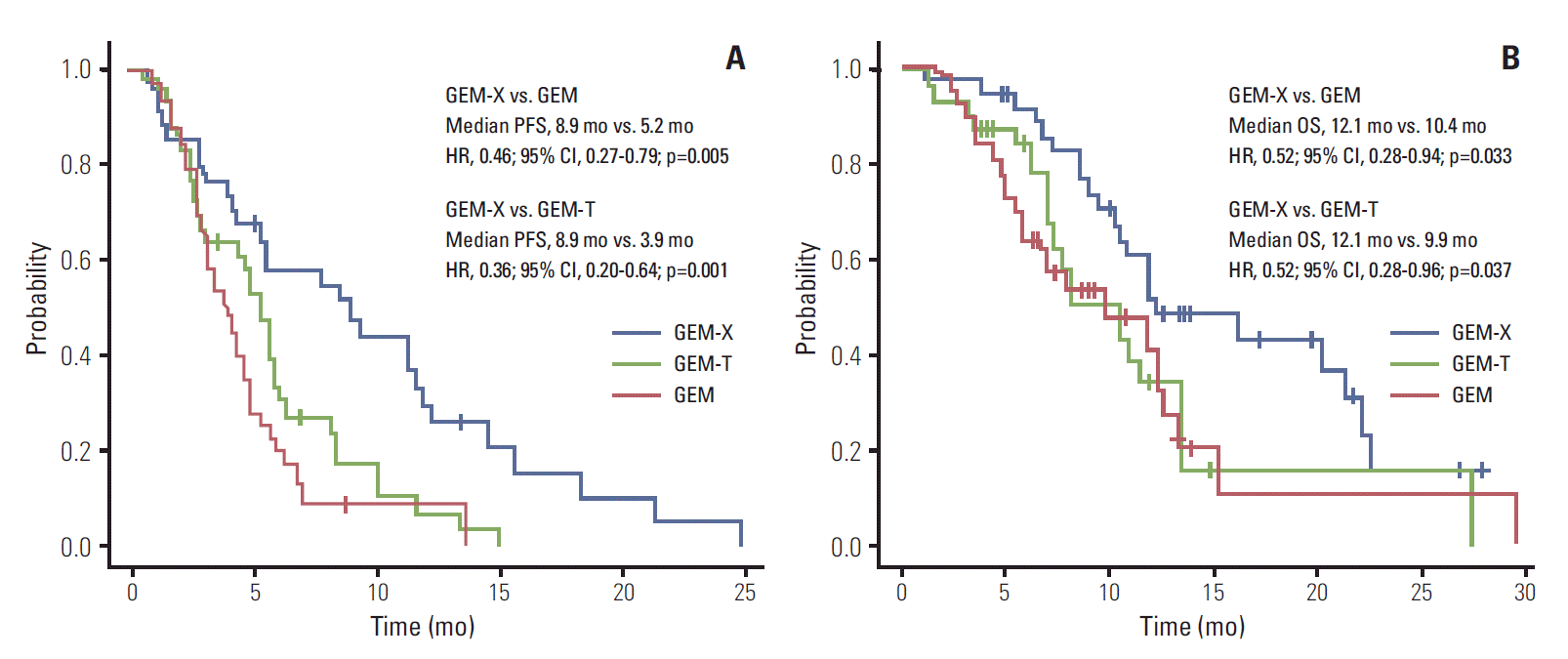
Table 1.
Patient characteristics
| Characteristic | Total (n=127) | GEM (n=47) | GEM-T (n=44) | GEM-X (n=36) |
|---|---|---|---|---|
| Age (yr) | ||||
| Median (range) | 65 (32-84) | 68 (41-84) | 63 (32-78) | 63 (38-77) |
| Gender | ||||
| Male | 72 (56.7) | 25 (53.2) | 25 (56.8) | 22 (61.1) |
| ECOG performance status | ||||
| 0 or 1 | 116 (91.3) | 41 (87.2) | 41 (93.2) | 34 (94.5) |
| 2 | 11 (8.7) | 6 (12.8) | 3 (6.8) | 2 (5.5) |
| Extent of disease | ||||
| Locally advanced | 46 (36.2) | 21 (44.7) | 11 (25) | 14 (38.9) |
| Distant metastatic | 68 (53.5) | 25 (53.2) | 26 (59.1) | 17 (47.2) |
| Recurred | 13 (10.3) | 1 (2.1) | 7 (15.9) | 5 (13.9) |
| CA 19-9 >500 U/mL | 64 (51.2) | 25 (54.3) | 23 (53.5) | 16 (44.4) |
| Primary site | ||||
| Head | 73 (57.5) | 30 (63.8) | 22 (50) | 21 (58.3) |
| Body | 36 (28.3) | 12 (25.5) | 14 (31.8) | 10 (27.8) |
| Tail | 14 (11) | 4 (8.5) | 6 (13.6) | 4 (11) |
| Total | 2 (1.5) | 1 (2.1) | 0 | 1 (2.8) |
| Metastatic site | ||||
| Liver | 49 (38.6) | 13 (27.7) | 21 (47.7) | 15 (41.7) |
| Peritoneum | 22 (17.3) | 9 (19.1) | 7 (15.9) | 6 (16.7) |
| Lung | 16 (12.6) | 11 (23.4) | 4 (9) | 1 (2.8) |
| Distant lymph node | 8 (6.3) | 3 (6.4) | 2 (4.5) | 3 (8.3) |
| Other | 7 (5.5) | 2 (4.2) | 4 (9) | 1 (2.8) |
| Prior therapy | ||||
| Chemotherapya) | 11 (8.7) | 0 (0) | 6 (13.6) | 5 (13.9) |
| Radiotherapy | 7 (5.5) | 1 (2.1) | 4 (9) | 2 (5.5) |
Table 2.
Tumor response
Table 3.
Hazard ratio of survival by pretreatment characteristics (GEM-X arm vs. GEM-T arm)
| Factor | No. |
PFS |
OS |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| GEM-X:GEM-T | 80 | 0.36 | 0.20-0.64 | 0.001a) | 0.52 | 0.28-0.96 | 0.037a) |
| Age (yr) | |||||||
| ≤65 | 46 | 0.36 | 0.16-0.81 | 0.013a) | 0.33 | 0.14-0.76 | 0.01a) |
| >65 | 34 | 0.24 | 0.08-0.68 | 0.007a) | 0.94 | 0.35-2.48 | 0.903 |
| ECOG performance status | |||||||
| 0 or 1 | 75 | 0.38 | 0.21-0.70 | 0.002a) | 0.57 | 0.30-1.10 | 0.095 |
| 2 | 5 | 0.01 | 0.00-153.5 | 0.36 | 0.01 | 0.00-153.5 | 0.36 |
| Disease status | |||||||
| Locally advanced | 25 | 0.25 | 0.07-0.85 | 0.027a) | 0.43 | 0.13-1.46 | 0.179 |
| Distant metastatic | 43 | 0.36 | 0.16-0.77 | 0.009a) | 0.45 | 0.19-1.07 | 0.074 |
| CA 19-9 (U/mL) | |||||||
| ≤500 | 40 | 0.27 | 0.11-0.62 | 0.002a) | 0.6 | 0.24-1.52 | 0.285 |
| >500 | 39 | 0.58 | 0.26-1.28 | 0.182 | 0.45 | 0.19-1.05 | 0.067 |
Table 4.
Toxicity and dosage modifications




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download