Abstract
We describe two cases of pulmonary arterial hypertension (PAH) that occurred under dasatinib treatment and were resolved after dasatinib discontinuation. Two patients with chronic phase chronic myeloid leukemia (CML) were switched to dasatinib therapy because of hematological progress while receiving imatinib. These patients had New York Heart Association (NYHA) functional class II dyspnea with elevated right ventricular systolic pressure (RVSP), which progressed under dasatinib treatment. After dasatinib treatment was discontinued, subjective symptoms were improved to NYHA functional class I and the follow-up transthoracic Doppler echocardiography showed improved RVSP. Treatment with an alternate tyrosine kinase inhibitor was initiated and had been continued without development of dyspnea or elevation of RVSP. This report suggests that dasatinib can cause the reversible PAH, therefore, routine cardiopulmonary evaluation before and during treatment with dasatinib may be needed in CML patients with clinical manifestations.
Pulmonary arterial hypertension (PAH) is a group of diseases characterized by progressive increase in pulmonary vascular resistance (PVR) (mean pulmonary arterial pressure [PAP] > 25 mm Hg at rest or > 30 mm Hg with exercise, as confirmed by right heart catheterization), leading to right ventricular failure and premature death [1]. Elevated PVR may result from multiple mechanisms, including vasoconstriction, proliferative and obstructive remodeling of pulmonary vessel wall, inflammation, and thrombosis [1]. PAH is a rare disease with an incidence of only 5.9 cases per 1 million, and primary PAH is an aggressive disease with a median survival of 2.8 years [2].
Dasatinib is an oral multi-target tyrosine kinase inhibitor of BCR-ABL, platelet-derived growth factor receptor (PDGFR), Src, discoidin domain receptor, and c-Kit and has a ~300-fold greater potency in ABL inhibition compared to imatinib in in vitro studies [3]. In 2010, dasatinib was approved by the Food and Drug Administration for treatment of chronic myeloid leukemia (CML) as a frontline therapy and as a second-line treatment after imatinib failure. However, previous case reports have identified several concerns over the development of PAH in dasatinib-treated CML patients [3-8]. Here, we describe two CML patients who developed PAH while receiving dasatinib treatment, but were improved shortly after discontinuation of dasatinib.
A 43-year-old male patient without comorbidity was diagnosed with chronic phase (CP) CML in 1999. He had been treated with imatinib until hematological progression; this patient was subsequently treated with a daily regimen of 140 mg dasatinib beginning from July 15, 2005. After several adjusted doses were administered because of repeated thrombocytopenia, the final dasatinib dose was restored in June 2010 to 140 mg daily and was maintained without additional adverse events (AEs).
On April 15, 2011, 69 months after initial dasatinib administration, the patient presented with New York Heart Association (NYHA) functional class II dyspnea. A chest radiograph revealed that the patient had grade 2 pleural effusion (PE). Furosemide (40 mg/day) and prednisolone (30 mg/day) were administered for 3 weeks. Although PE improved with this treatment, the patient’s dyspnea persisted to the same degree. Electrocardiography showed slight right ventricular hypertrophy. A transthoracic Doppler echocardiogram revealed a highly increased right ventricular systolic pressure (RVSP) of 92 mm Hg, an enlarged right heart cavity (right atrial area of 37.8 cm2, basal right ventricular end-diastolic diameter of 44.5 mm), and a small amount of pericardial effusion. The left heart chambers and left ventricular ejection fraction were normal. The systolic PAP was estimated at 90 mm Hg, and right ventricular function was impaired, as assessed by a reduced tricuspid annular plane systolic excursion of 15.6 mm. Other potential causes of PAH (such as congenital heart disease, pulmonary embolism, and parenchymal lung disease [1]) were ruled out by chest contrast-enhanced 3-dimensional computed tomography. B-type natriuretic peptide (BNP) levels were elevated to 353.33 pg/mL. No causative medication had been taken.
On May 9, 2011, dasatinib was discontinued, and a regimen of sildenafil, a calcium channel blocker, and diuretics was initiated. One month later, subjective symptoms improved to NYHA functional class I. Follow-up echocardiography revealed an improved RVSP of 79 mm Hg and an estimated systolic PAP of 80 mm Hg. Re-evaluation revealed a significant improvement, with RVSP decreased to 57 mm Hg and PE completely absent 2 months after dasatinib discontinuation. On July 4, 2011, the patient started ponatinib at a dose of 45 mg once daily, and this treatment has been continued without any AEs. The changes in clinical course, cardiopulmonary parameters and treatment history are depicted in Fig. 1A and C and Table 1.
A 52-year-old male patient was diagnosed with CP CML in 2001. He was initially treated with interferon-alpha and later received a daily regimen of 400 mg imatinib that was dose-adjusted over time because of AEs. He had pleurisy of unknown origin during his teenage years. Because no cytogenetic response was achieved on imatinib, dasatinib was initiated at a dose of 140 mg daily from December 2, 2005 and was initially well tolerated.
However, in February 2008, the patient complained of intermittent NYHA class II dyspnea on effort, and his chest radiograph revealed right PE; with sustained dasatinib treatment, the symptom progressed to NYHA class III on February 24, 2009. At that time, dasatinib was discontinued, and treatment with low dose sildenafil (25 mg/day) was initiated. One month after dasatinib discontinuation, a chest radiograph revealed a nearly resolved state. However, a chest computed tomography scan revealed mild pulmonary congestion, and a transthoracic Doppler echocardiogram showed an increased RVSP of 71 mm Hg and a small amount of pericardial effusion. BNP levels were elevated to 263 pg/mL. Laboratory tests for human immunodeficiency virus (HIV) and rheumatic diseases, including antinuclear antibodies, antibodies to double-stranded DNA, and anti-Scl-70 antibodies, were negative. A careful review of concurrent medications or toxins suspected to play an essential role in the development of PAH revealed no causative evidence.
After switching to nilotinib (800 mg/day) in May 2009, the patient’s subjective dyspnea improved, and his RVSP decreased to 55 mm Hg. BNP levels returned to 28 pg/mL, and the 6-minute-walk test value improved from 480 m to 615 m. The patient intermittently discontinued nilotinib because of repeated hyperbilirubinemias and was transferred to our hospital in July 2009. Eighteen months later, he lost cytogenetic response with an E292V mutation; therefore, a daily dose of 100 mg dasatinib was reintroduced in December 2010. Although the patient previously experienced dasatinib-associated PAH, we chose to administer dasatinib in order to overcome the patient’s mutation-related resistance. The dose of dasatinib (40-60 mg/day) was then adjusted according to the severity of dyspnea, and a major cytogenetic response on dasatinib was maintained.
After dasatinib administration, the patient again experienced a sustained NYHA functional class II dyspnea with serial elevation of left ventricular systolic pressure observed in a follow-up echocardiogram (Fig. 1B). On April 11, 2011, a daily dose of 45 mg ponatinib was prescribed without improvement in PAH while maintaining a complete cytogenetic response and a major molecular response. In August 2012, the patient's subjective dyspnea was improved to NYHA functional class I. The changes in clinical course, cardiopulmonary parameters and treatment history are depicted in Fig. 1B and Table 1.
The cause of PAH can be classified as idiopathic, hereditary, or associated with congenital heart disease, connective tissue disease, HIV infection, portal hypertension, drugs and toxins, and hemoglobinopathies [1]. Although right heart catheterization was not performed to establish a diagnosis of PAH in our two patients, they had an estimated systolic PAP of over 30 mm Hg in echocardiogram. These patients did not have any possible causes of PAH such as family history of PAH, congenital heart disease, infection, rheumatologic disease or HIV.
PAH in our patients continued to worsen during administration of dasatinib but became normalized after switching to another tyrosine kinase inhibitor. According to French reports, the lowest estimated incidence of PAH occurring in patients exposed to dasatinib was 13 of 2,900 (0.45%) [9]. The potential mechanism of PAH in patients treated with dasatinib is currently unclear. Preclinical findings have suggested that an imbalance in PDGFR expression contributes to excessive smooth muscle proliferation observed in ovine perinatal pulmonary hypertension [10]. Another study found that platelet-derived growth factor (PDGF) and PDGFR mRNA are overexpressed in pulmonary arterial smooth muscle cells and endothelial cells from the pulmonary arteries of patients displaying severe PAH; in addition, immunostaining of these tissues confirms high protein levels of PDGF/PDGFR [11].
The ability to inhibit PDGFR signaling suggests that both imatinib and dasatinib have similar effects on pulmonary vascular remodeling; furthermore, imatinib was effective for the treatment of PAH through blockade of PDGFR phosphorylation in several studies [12]. Therefore, a unique mechanism of dasatinib-associated PAH may exist independent of effects on PDGF/PDGFR signaling. The main difference in the kinase profile between dasatinib and imatinib is the inhibition of Src family kinases [13]. Preclinical findings suggest that Src kinase activity is related to hypoxic vasoconstriction in the rat pulmonary artery [13]. Therefore, Src activity may be one of the main mechanisms of dasatinibrelated PAH.
The patients presented in this report and other patients in some previously published cases have several common characteristics (Table 2). First, dasatinib had been given for a long period of time (19-52 months) before clinical manifestations of PAH developed. The occurrence of PAH at a late onset suggests a chronic pathological mechanism with an insidious, as opposed to acute, inflammatory, or cardiac etiology [14]. In our case, the first patient developed dyspnea after approximately 69 months on dasatinib, and the second patient developed dyspnea after 38 months in the first instance and almost immediately after starting dasatinib in the second instance. For the second patient, during the second administration of dasatinib, we observed a short time interval between the initiation of dasatinib and diagnosis of PAH. Given that this was a re-trial of dasatinib and his baseline RVSP was already elevated to 55 mm Hg at the time of restarting dasatinib, the short interval between initiation of dasatinib and manifestation of PAH symptoms can be explained. Second, these patients demonstrated rapid symptomatic and hemodynamic improvement (within several days to 4 months) after discontinuation of dasatinib. This reversibility is remarkable because normalization of pulmonary hemodynamics is not usually achieved in PAH with usual medical treatments [3]. Third, a majority of patients have had a history of PE with pericardial effusion. It was previously reported that PE occurred in 17%-54% of patients on dasatinib, which suggests a similar mechanism of development for dasatinib-associated PAH and PE [14,15]. Given these common characteristics, we suggest that dasatinib is the primary cause of PAH in our two cases.
In conclusion, long-term treatment with dasatinib may contribute to PAH, which may improve spontaneously after discontinuation. Routine cardiopulmonary evaluation before and during treatment with dasatinib will be needed in patients with clinical manifestations.
References
1. Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009; 30:2493–537.
2. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006; 173:1023–30.
3. Dumitrescu D, Seck C, ten Freyhaus H, Gerhardt F, Erdmann E, Rosenkranz S. Fully reversible pulmonary arterial hypertension associated with dasatinib treatment for chronic myeloid leukaemia. Eur Respir J. 2011; 38:218–20.

4. Rasheed W, Flaim B, Seymour JF. Reversible severe pulmonary hypertension secondary to dasatinib in a patient with chronic myeloid leukemia. Leuk Res. 2009; 33:861–4.

5. Orlandi EM, Rocca B, Pazzano AS, Ghio S. Reversible pulmonary arterial hypertension likely related to long-term, low-dose dasatinib treatment for chronic myeloid leukaemia. Leuk Res. 2012; 36:e4-6.

6. Mattei D, Feola M, Orzan F, Mordini N, Rapezzi D, Gallamini A. Reversible dasatinib-induced pulmonary arterial hypertension and right ventricle failure in a previously allografted CML patient. Bone Marrow Transplant. 2009; 43:967–8.

7. Sano M, Saotome M, Urushida T, Katoh H, Satoh H, Ohnishi K, et al. Pulmonary arterial hypertension caused by treatment with dasatinib for chronic myeloid leukemia: critical alert. Intern Med. 2012; 51:2337–40.

8. Hennigs JK, Keller G, Baumann HJ, Honecker F, Kluge S, Bokemeyer C, et al. Multi tyrosine kinase inhibitor dasatinib as novel cause of severe pre-capillary pulmonary hypertension? BMC Pulm Med. 2011; 11:30.

9. Montani D, Bergot E, Gunther S, Savale L, Bergeron A, Bourdin A, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012; 125:2128–37.
10. Balasubramaniam V, Le Cras TD, Ivy DD, Grover TR, Kinsella JP, Abman SH. Role of platelet-derived growth factor in vascular remodeling during pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2003; 284:L826–33.
11. Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008; 178:81–8.

12. Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. 2010; 182:1171–7.

13. Pullamsetti SS, Berghausen EM, Dabral S, Tretyn A, Butrous E, Savai R, et al. Role of Src tyrosine kinases in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2012; 32:1354–65.

14. Quintas-Cardama A, Kantarjian H, O'Brien S, Borthakur G, Bruzzi J, Munden R, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007; 25:3908–14.
Fig. 1.
(A) The changes in clinical courses, cardiopulmonary parameters and treatment history in patient in case 1 are depicted over time. (B) The changes in parameters of in patient in case 2 are depicted over time. (C) Patient in case 1 had an elevated tricuspid regurgitation velocity (4.4 m/sec), consistent with pulmonary arterial hypertension (left). Four months after dasatinib discontinuation (right), there was an improvement of tricuspid regurgitation velocity (3.6 m/sec) in patient 1. NYHA, New York Heart Association; RVSP, right ventricular systolic pressure; G, grade; SF, sildenafil; TKI, thyrosine kinase inhibitor; HUR, hydroxyurea; IM, imatinib; Das, dasatinib; Pon, ponatinib; HR, hematologic response; CP, chronic phase; CHR, complete hematologic response; IS, international scale; Nil, nilitinib.
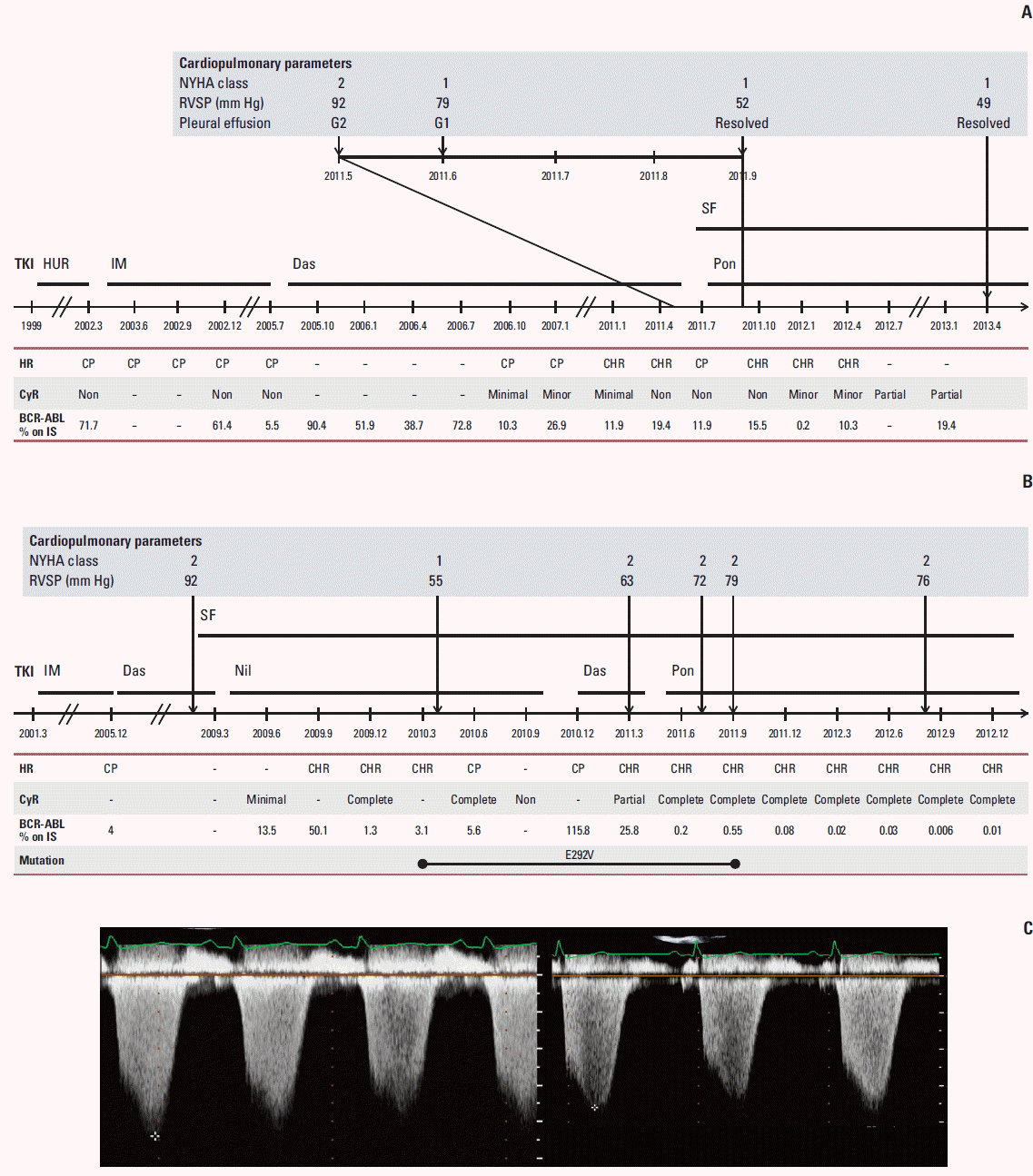
Table 1.
The change of parameters in non-invasive evaluation during the course of the treatment with dasatinib
Table 2.
Characteristics of previously reported CML patients with dasatinib-associated PAH
| Source | Age at diagnosis (yr) | Gender | Delay between initiation of dasatinib and diagnosis of PAH (mo) | Delay between discontinuation of dasatinib and symptom improvement (mo) | Existence of pleural effusion at diagnosis | Dose of dasatinib at diagnosis (mg/day) | Concomitant treatment of PAH | Previous therapy for CML |
|---|---|---|---|---|---|---|---|---|
| Mattel et al. (2009) [6] | 48 | M | 19 | 4 | Present | 50 | Furosemide, pulse steroid | Interferon-alpha, HSCT, imatinib |
| Rasheed et al. (2009) [4] | 41 | M | 26 | 2.5 | Present | 140 | - | Hydroxyurea, imatinib |
| Dumitrescu et al. (2011) [3] | 47 | M | 52 | 2 | Present | 100 | Sildenafil | Interferon-alpha, imatinib |
| Hennings et al. (2011) [8] | 70 | M | 32 | Within days | Present | 140 | Sildenafil | Hydroxyurea with interferon-alpha, imatinib |
| Orlandi et al. (2012) [5] | 53 | F | 31 | 1 | Absent | 70 | Furosemide | Imatinib |
| Sano et al. (2012) [7] | 61 | F | 27 | 4 | Present | 140 | Sildenafil | Imatinib |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download