Abstract
We report on a patient with brain metastasis involving bilateral internal auditory canal from non-small cell lung cancer (NSCLC). A 49-year-old woman who had been diagnosed with NSCLC (T2aN1M0) complained of persistent vertigo and bilateral tinnitus for three months. The patient had refused all treatments, including surgery and chemotherapy; however, she sought alternative medicine. The patient’s hearing loss showed rapid progression bilaterally, and rotatory vertigo with peripheral-type nystagmus developed. Magnetic resonance imaging of the brain showed irregular nodular enhancement within both internal auditory canals with leptomeningeal enhancement and multiple intracranial metastasis. The patient was treated with epidermal growth factor receptor-tyrosine kinase inhibitor, and the tumor showed partial response. This was a rare case of multiple brain metastases involving bilateral internal auditory canal from known NSCLC presenting with vertigo and hearing loss.
Lung cancer is one of the most common fatal malignancies [1]. Pulmonary adenocarcinoma, which constitutes approximately 35% of lung cancer, has been reported to cause brain metastasis in up to 28% of cases [2,3]. Metastasis to the temporal bone from the pulmonary adenocarcinoma is not uncommon, and solitary metastasis to the internal auditory canal (IAC) has been reported [4,5]. However, metastasis to bilateral IAC from primary lung cancer has rarely been reported. We report on a patient with multiple intracranial metastases involving bilateral IAC from pulmonary adenocarcinoma.
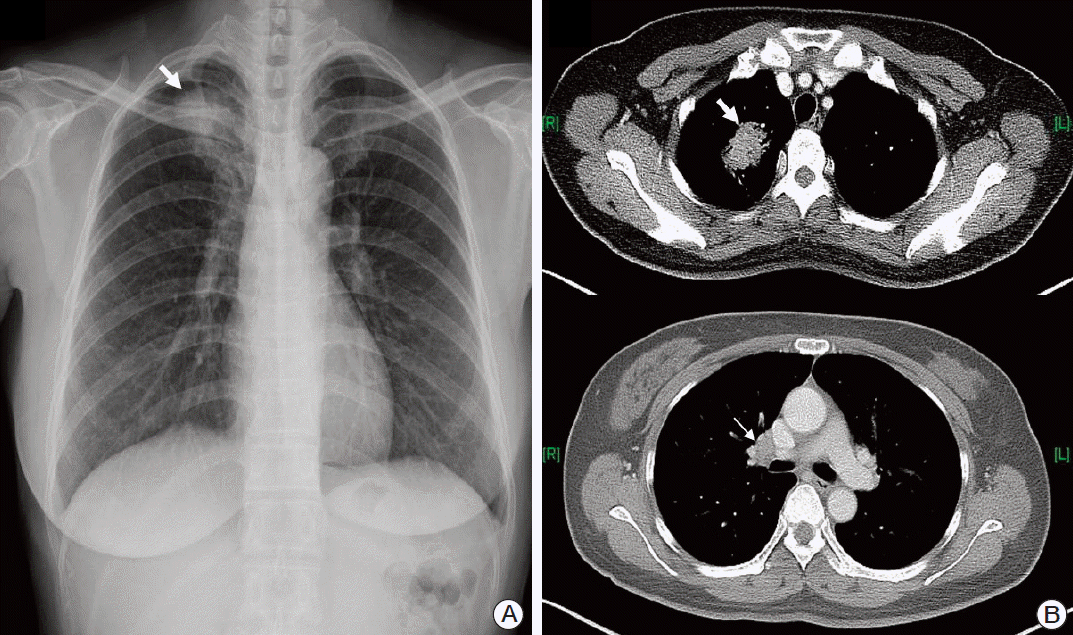
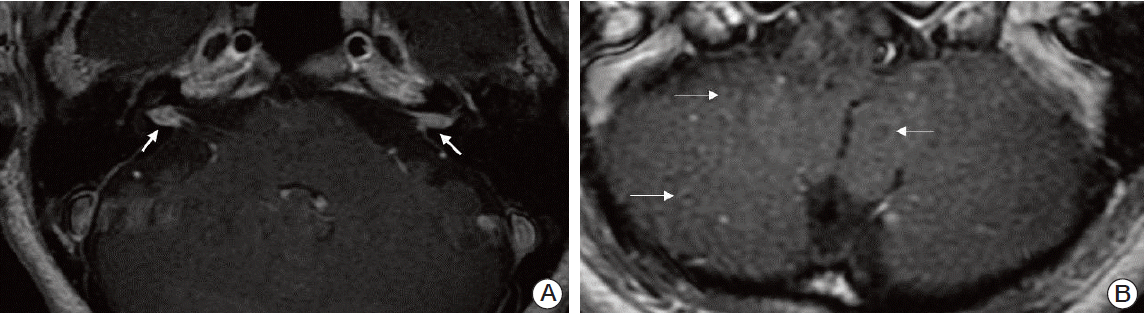
A 49-year-old female patient came to our outpatient department with a 3-month history of continuous vertigo. She also presented with rapidly deteriorating hearing loss and tinnitus on both sides. The patient exhibited left-beating spontaneous nystagmus, and head impulse test showed a catch-up saccade on the right side. She did not report pain in the mastoid or retromastoid area. Except for headache, no other neurologic symptoms, including facial nerve paralysis, were observed. Pure tone audiometry (PTA) showed sensorineural hearing loss on both sides (Fig. 1A), and bithermal caloric test showed 75% canal paresis on the right side (Fig. 1B). The patient had been diagnosed four months ago with non-small cell lung cancer (NSCLC, T2aN1M0) at another hospital (Fig. 2). She refused any treatment, including surgery and/or chemotherapy, and has been under the care of alternative medicine. Magnetic resonance imaging (MRI) of the brain showed irregular nodular enhancement within both IAC with leptomeningeal enhancement and multiple tiny rim enhancing parenchymal metastases (Fig. 3). Cerebrospinal fluid (CSF) was clear and colorless. CSF analysis showed the following results: white blood cell 0/μL and red blood cell 970/μL, protein 71.5 mg/dL, and glucose 51 mg/dL. Although cytology was also negative for any malignant cells, protein and the carcinoembryonic antigen (CEA) level (95.69 ng/mL) was higher than normal range at the initial diagnosis. After disease progression, the CEA level (459.0 ng/mL) had more than quadrupled and the CSF cytology result was suspicious for malignant cells central nervous system symptoms, an elevated CEA [6], and abnormal MRI findings were consistent with leptomeningeal carcinomatosis. We confirmed stage IV (T3N1M1b) adenocarcinoma with activating epidermal growth factor receptor (EGFR) mutation, exon 21 L858R. Subsequently, she received gefitinib as palliative treatment for primary and metastatic tumor and palliative whole brain radiation therapy to a total dose of 30 Gy in 10 fractions. The tumor showed partial response (Fig. 4). However, the patient’s hearing showed progressive deterioration despite chemotherapy, and PTA showed total deafness on both sides. Impairment of bilateral vestibular function had also progressed. The patient complained of aggravation of oscillopsia, and head impulse test showed bilateral catch-up saccade, indicating bilateral vestibulopathy.
The vast majority of lesions of the cerebellopontine angle (CPA) and IAC are benign tumors such as vestibular schwannoma and meningioma [7]. Metastasis to IAC has rarely been reported and accounts for only 0.3% of CPA lesions. The most common origins of metastasis to the temporal bone are breast cancer, lung cancer, renal cancer, stomach cancer, and prostate cancer [8]. Hematogenous dissemination, leptomeningeal carcinomatosis, and direct extension from the adjacent areas are considered as possible routes to temporal bone metastasis, and hematogenous spread accounts for most cases of brain metastasis. Bilateral IAC involvement was reported to occur in almost half of patients with IAC metastasis [8]. The route of metastasis into IAC is still unclear, but spread of metastatic lesions into meninges and CSF is generally considered the mechanism of tumor deposits in IAC. Even distribution of malignant cells bilaterally in the CSF can lead to bilateral IAC involvement [8].
Facial nerve palsy was reported as the most common symptom of bilateral IAC metastasis, and facial paralysis on at least one side was observed in all patients with bilateral IAC lesions [8]. While hearing loss was observed in 71% (10/14) of patients with bilateral IAC metastasis, only 21% (3/14) of patients complained of vertigo or disequilibrium [8]. Persistent vertigo and bilateral tinnitus were the chief complaint of our patient, whereas facial paralysis was not observed. While bilateral sensorineural hearing loss was revealed on PTA, caloric response showed canal paresis on the right side, even though the size of metastatic nodules was comparable bilaterally. It was reported that facial paralysis may not be observed even though the facial nerve was infiltrated with metastatic cancer cells in IAC [9].
In our patient, the suspicion of IAC metastasis was not difficult because lung adenocarcinoma is one of the most common primary sources of brain metastasis, and the patient did not receive any treatment for three months since the diagnosis of the disease. However, differential diagnosis between IAC metastasis and primary benign tumors such as vestibular schwannoma is often difficult. Clinical history of rapidly progressing sensorineural hearing loss accompanied by severe facial paralysis might raise the possibility of IAC metastasis, even though 30% of patients with unilateral IAC metastasis can be asymptomatic [8,10]. It has been reported that a lack of distinctive features on brain MRI in IAC metastasis makes differentiation from benign tumors more difficult [10]. In general, vestibular schwannoma shows a homogeneous and isointense signal compared to gray matter on both T1- and T2-weighted images, and exhibits strong postgadolinium enhancement. The presence of thick linear and extranodular contrast enhancement on MRI may favor metastasis [11]. MRI findings of irregular nodular enhancement within the IAC with leptomeningeal enhancement, as shown in the current patient, may be helpful in the differential diagnosis.
The prognosis of leptomeningeal carcinomatosis from NSCLC is poor. There are several palliative therapeutic options, such as intrathecal and/or intravenous chemotherapy, radiation therapy, and ventriculoperitoneal shunt, but they would have little effect on survival. However, EGFRtyrosine kinase inhibitor (TKI) would be a favorable treatment option in patients who harbor activating EGFR mutation regardless of leptomeningeal carcinomatosis [12,13]. Our patient showed a similar response to EGFR-TKI; she showed a partial response after first-line gefitinib, with progression-free survival of five months. While the tumor progressed after one cycle of second-line pemetrexed, reduction in tumor volume was observed after the patient received third-line erlotinib.
Thorough history taking and physical examination are crucial in diagnosis of metastatic tumor of IAC, and a high index of suspicion would enhance the correct diagnosis since distinctive radiological features of IAC metastasis may not be observed on MRI. Attention should be paid when patients who have or had primary malignancy with high incidence of brain metastasis show symptoms such as vertigo, hearing loss, tinnitus, and facial nerve palsy with rapid progression or sudden onset.
References
2. Little AG, Gay EG, Gaspar LE, Stewart AK. National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer. 2007; 57:253–60.

3. Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988; 6:1474–80.
4. Chang KH, Song CE, Seo JH, Yeo SW. Solitary metastasis of bronchogenic adenocarcinoma to the internal auditory canal: a case report. J Korean Med Sci. 2009; 24:1227–9.

5. Schrock A, Laffers W, Bootz F. Solitary metastasis of lung carcinoma to the internal auditory canal. Am J Otolaryngol. 2006; 27:214–6.

6. Kang SJ, Kim KS, Ha YS, Huh SY, Lee JH, Kim JK, et al. Diagnostic value of cerebrospinal fluid level of carcinoembryonic antigen in patients with leptomeningeal carcinomatous metastasis. J Clin Neurol. 2010; 6:33–7.

7. Moffat DA, Saunders JE, McElveen JT Jr, McFerran DJ, Hardy DG. Unusual cerebello-pontine angle tumours. J Laryngol Otol. 1993; 107:1087–98.

8. Streitmann MJ, Sismanis A. Metastatic carcinoma of the temporal bone. Am J Otol. 1996; 17:780–3.
9. Yoda S, Cureoglu S, Paparella MM. Pulmonary carcinoma metastasis to the internal auditory canal. Otol Neurotol. 2011; 32:e48–9.

10. Loo SW, Dean AF, Murray P. Internal auditory canal metastasis mimicking a vestibular schwannoma at presentation: a case report and review of the literature. Int Semin Surg Oncol. 2009; 6:8.

11. Krainik A, Cyna-Gorse F, Bouccara D, Cazals-Hatem D, Vilgrain V, Denys A, et al. MRI of unusual lesions in the internal auditory canal. Neuroradiology. 2001; 43:52–7.

Fig. 1.
Pure tone audiometry shows sensorineural hearing loss on both sides (A), and bithermal caloric test shows canal paresis of 75% on the right side (B). SPV, slow phase velocity.
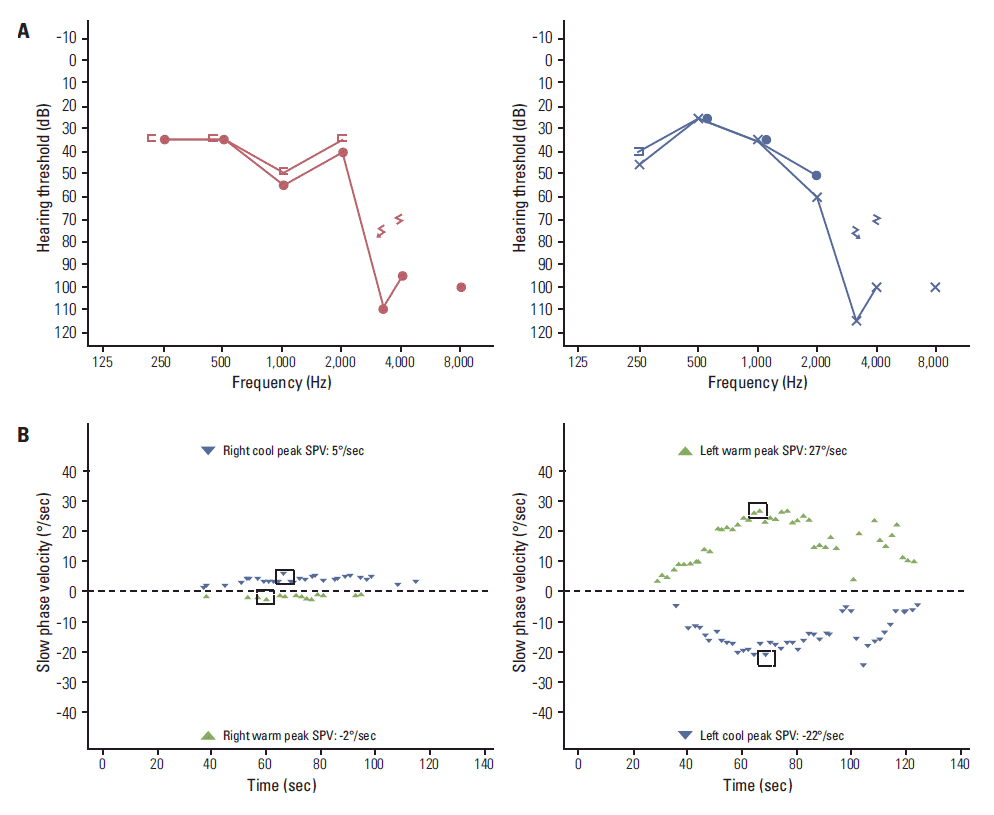
Fig. 2.
(A) Chest X-ray shows a mass lesion in the right upper lobe (RUL) (arrow). (B) Computed tomography of the chest shows a 4.2-cm mass (thick arrow) in RUL with enlargement of ipsilateral hilar lymph nodes (thin arrow).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download