Abstract
Radiation-induced hemorrhagic gastritis is an intractable and dangerous condition. We describe a 59-year-old female patient with radiation-induced hemorrhagic gastritis. The patient underwent postoperative radiation therapy with a dose of 54 Gy in 30 fractions after a radical operation for a Klatskin tumor. Radiation volume included the gastric antrum. Approximately three months after radiation therapy, she was admitted for melena and anemia. Esophagogastroduodenoscopy showed an area of bleeding in the gastric antrum that was so diffuse that effective laser coagulation was not feasible. After failure of various treatments and transfusion of 7,040 mL of packed red blood cells, we successfully stopped the hemorrhage using oral prednisolone treatment. Based on this case, we think that oral prednisolone treatment can be tried as a first treatment for potentially life-threatening radiation-induced hemorrhagic gastritis.
Radiation-induced hemorrhagic gastritis is a serious complication and is very difficult to manage. Although various treatments are available, there is no established gold standard of treatment. We successfully treated a case of intractable gastric bleeding induced by postoperative radiation using oral prednisolone.
A 59-year-old woman presented with dark urine and generalized pruritus in April 2012. Diagnostic work up revealed a stage IIIB or IV Klatskin tumor. Previous to this, she had undergone total abdominal hysterectomy and bilateral salpingoophorectomy for uterine cervical cancer in February 1993 and had undergone a modified radical left mastectomy for left-sided breast cancer in September 2008. Thus, the Klatskin tumor was her third cancer.
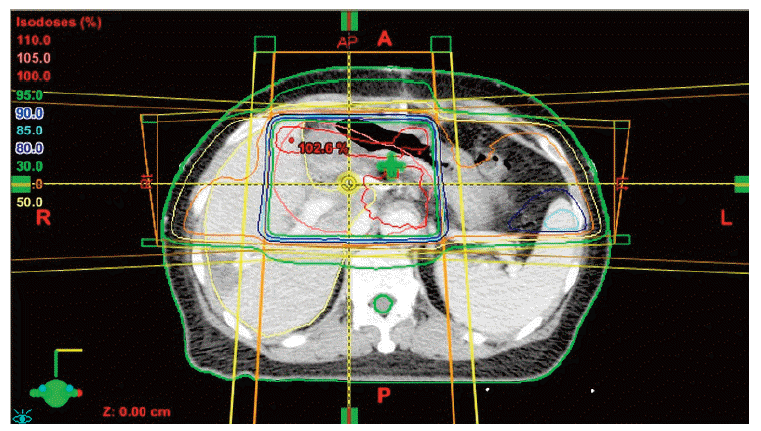
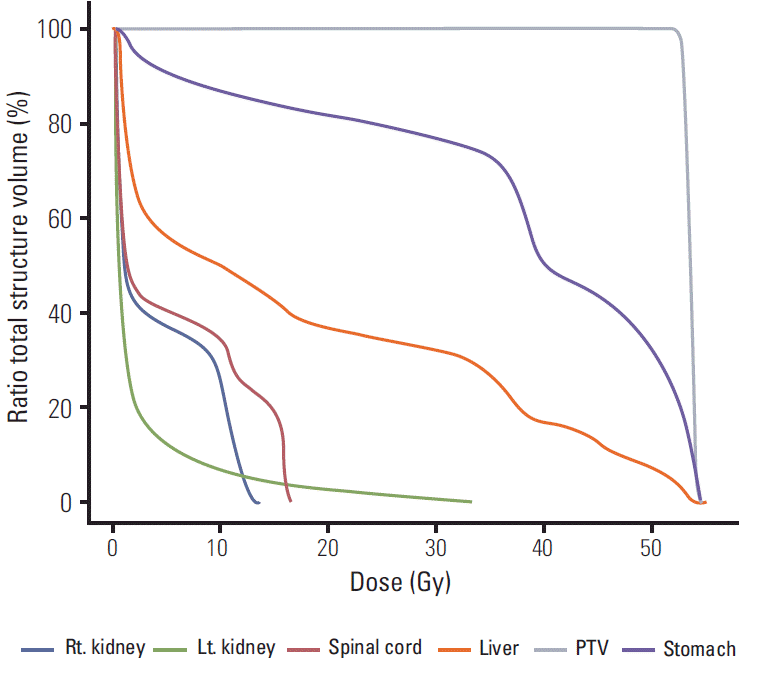
After confirmation of the Klatskin tumor, a radical operation was performed on April 20, 2012. The operation consisted of resection of the left lobe of the liver and radical extrahepatic bile duct resection with Roux-en-Y heapticojejunostomy, combined with portal vein partial resection and re-anastomosis. The tumor was pathologically diagnosed as adenocarcinoma consistent with a Klatskin tumor that had directly invaded the liver. In addition, the tumor was present in the portal vein, lymphatic area, cystic duct, and perineural area. The distal bile duct resection margin was focally involved, and the liver parenchymal resection margin was very close. Because of these high risk pathological findings, postoperative three dimensional conformal radiotherapy was performed from May 22, 2012, to July 3, 2012, with a total radiation dose of 54 Gy over 30 fractions. The radiation treatment volume included the tumor bed and regional lymph node area. The antrum of the stomach was also included in the radiation treatment volume (Fig. 1). Mean radiation dose of the stomach was 37.7 Gy, and the maximum radiation dose to the stomach antrum was 55.1 Gy. In the dose volume histogram (Fig. 2), 30% of stomach volume received a radiation dose higher than 50 Gy, and the stomach antrum received a greater part of the overall radiation burden to the stomach. Cisplatin and 5-fluorouracil systemic chemotherapy was administered concurrently with radiation therapy. After the end of radiation therapy, chemotherapy was continued for a total of six cycles. The last chemotherapy cycle ended on October 12, 2012.
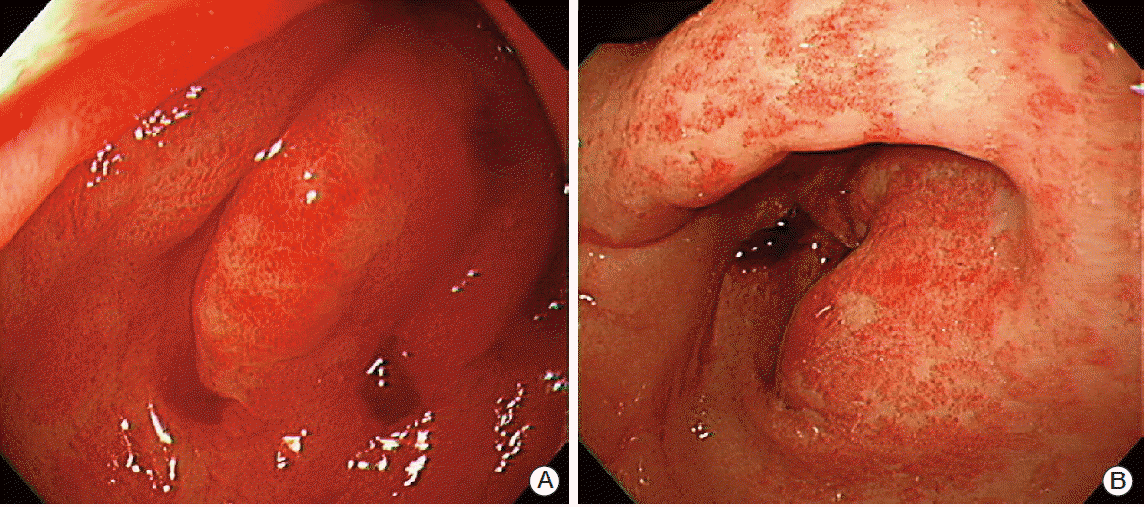
The patient was admitted on November 13, 2012 (100 days after completing radiotherapy) with melena and anemia. Her platelet count (115×103/μL) was consistent with a grade 1 thrombocytopenia. The results of blood coagulation tests (prothrombin time [PT] international normalization ratio 1.11, PT % 80.0, PT sec 11.8, activated partial thromboplastin time 35.7 seconds) were normal. She did not take any anticoagulant or antiplatelet medications, and did not have a history of bleeding disorder. Esophagogastrodudenoscopy (EGD) showed diffuse edematous antral hyperemia and bleeding (Fig. 3A). The oozing area was so diffuse that effective laser coagulation was not feasible. On abdominopelvic computed tomography, no bleeding focus was found throughout the small and large bowels or at the anastomosis site. A proton pump inhibitor was initiated at admission, but the melena persisted. Between November 13 and 15, the patient required 2,240 mL of packed red blood cells (RBC). Follow-up EGD performed on November 19 showed no change in hemorrhagic antral mucosa, during which epinephrine was injected. However, the melena persisted, and transfusion of 3,200 mL of additional packed RBC was performed between November 20 and 30. Because the area of bleeding was confined to the highly irradiated antral area and various measures were not successful, we concluded that the hemorrhagic gastritis was due to radiation therapy. Therefore, we decided to try oral steroid treatment, which (10 mg prednisolone four times/day; total daily dose 40 mg) was initiated on November 30. The melena stopped after six days of treatment with oral prednisolone without correction of thrombocytopenia. Between December 1 and 5, an additional 1,600 mL of packed RBC was transfused. On treatment day 8, the prednisolone dose was tapered to 20 mg daily (5 mg four times/day). EGD performed on December 10, 2012 (treatment day 11) showed discrete blood clots and scanty hemorrhagic mucosa in the pyloric antrum without a definite bleeding focus and markedly decreased oozing compared to the previous endoscopic study (Fig. 3B). The daily steroid dose was tapered to 10 mg (5 mg bid) on treatment day 11. After treatment day 14, the daily dose was tapered to 5 mg and maintained for two months. For the month following cessation of melena, a total of 1,120 mL RBCs were transfused. Since then, the patient has not experienced subsequent bleeding episodes, and did not require additional transfusion.
Severe gastric complications due to radiotherapy are uncommon, particularly for hemorrhagic gastritis. Yoon et al. [1] reported on three patients with National Cancer Institute (NCI) common toxicity criteria grade III or higher gastrointestinal bleeding or ulceration among 51 patients who underwent radiation therapy for treatment of lymph node metastases from hepatocellular carcinoma. Radiationinduced hemorrhagic gastritis can lead to significant gastric bleeding and is usually refractory to treatment, which makes it a potentially lethal complication. Successful treatment outcomes for patients with radiation-induced hemorrhagic gastritis are presented only as case reports due to the rarity of this complication and the refractoriness to treatment. High total doses and fractional dose appear to be the main risk factors for gastric injuries and may be potentiated by the concurrent administration of fluorouracil [2]. The patient in the current case had received a relatively high dose of radiation for the positive surgical resection margin, with concurrent administration of 5-fluorouracil.
Injury to the stomach from radiation therapy can result in a form of hemorrhagic gastritis, characterized by acute vasculopathy, which may progress to obliterative endarteritis with mucosal ischemia and ulceration. Normal tissue in proximity to a targeted neoplasm may develop ballooning degeneration with ischemia and cell death. Hyperbaric oxygen therapy can serve as a stimulus for development of neovascularity in these tissues [3]. A few cases of successful hyperbaric oxygen treatment have been reported [3,4], however, hyperbaric oxygen therapy is not widely available. Laser coagulation of the hemorrhagic gastric mucosa is another treatment option. Shukuwa et al. [5] reported successful use of argon plasma coagulation (APC) for hemostasis of widely spreading radiation hemorrhagic gastritis. The severe anemia showed improvement without cicatricial stenosis. As the application of APC must be limited to the surface mucosa, those authors suggested that APC is an easy and effective treatment for radiation hemorrhagic gastritis. Furukawa et al. [6] also reported on a patient with hemorrhagic gastritis induced by external radiotherapy for pancreatic cancer who was treated successfully using APC. Wada et al. [7] also described a case of effective treatment with APC in a similar patient. Unfortunately, in our patient, the bleeding from antral mucosa was diffuse, and laser coagulation was ineffective. On the other hand, Staiano et al. [8] reported successful treatment of radiation-induced hemorrhagic gastritis using endoscopic band ligation. Because conservative treatment is usually ineffective, gastrointestinal radiation injuries are often treated surgically. Flobert et al. [9] reported a case of hemorrhagic gastritis induced by radiotherapy, which was successfully treated with total gastrectomy. Yeung et al. [10] also reported a case of recurrent hemorrhagic gastritis successfully treated by surgical resection. Our patient was very fragile after suffering three major operations for three different cancers during her lifetime; thus, surgical resection of a heavily treated stomach appeared to be difficult and dangerous.
Because bleeding persisted despite various measurements and because of large amounts of transfusion required, spontaneous healing was not likely in this patient. We tried oral prednisolone treatment, as the effect of steroid is well known for radiation-induced inflammation in radiation pneumonitis and severe radiation proctitis. In our case, the melena showed dramatic improvement after only six days of treatment with oral prednisolone without correction of thrombocytopenia. Inaba et al. [11] reported a case of hemorrhagic radiation gastritis after chemoradiation therapy for peritoneal lymph node metastasis of esophageal carcinoma, which was successfully treated with repeated intra-arterial steroid infusions. Zhang et al. [12] recently reported a case of hemorrhagic radiation gastritis successfully treated with oral prednisolone, with outcomes similar to those of our case.
Although the exact nature of radiation-induced gastritis is not known, it is presumed to be an inflammatory process resulting in mucositis. Steroid is thought to have an antiinflammatory action by preventing the liberation of arachidonic acid from cell membranes, decreasing neutrophilic phagocytosis and by diminishing adherence and chemotaxis of neutrophils, eosinophils, and monocytes, subsequently leading to inhibitions of the release of other inflammatory cytokines. A reduction in neutrophil recruitment and local cytokine production, and improvements in microcirculation by reversal of capillary permeability have been proposed as mechanisms responsible for the effects of steroid in inflammatory bowel disease [13]. Indaram et al. [14] reported that the mucosal levels of cytokines such as interleukin (IL)-2, IL-6, and IL-8 were significantly increased in the colon among patients with radiation proctitis. Anti-inflammatory functions of prednisolone may reduce gastric mucosal damage and degeneration as well.
Based on this case, we believe that oral prednisolone treatment can be tried as a first treatment for potentially life threatening radiation-induced hemorrhagic gastritis. We expect that more case reports of treatment success for this refractory complication will substantiate our results in the future.
References
1. Yoon SM, Kim JH, Choi EK, Ahn SD, Lee SW, Yi BY, et al. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Res Treat. 2004; 36:79–84.

2. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991; 21:109–22.

3. Kernstine KH, Greensmith JE, Johlin FC, Funk GF, De Armond DT, Van Natta TL, et al. Hyperbaric oxygen treatment of hemorrhagic radiation-induced gastritis after esophagectomy. Ann Thorac Surg. 2005; 80:1115–7.

4. Banerjee N, Javed A, Deepak D, Pawar M, Chattopadhyay TK. Hyperbaric oxygen therapy: an adjuvant treatment modality for chemo-radiation induced hemorrhagic gastritis. Trop Gastroenterol. 2011; 32:248–50.
5. Shukuwa K, Kume K, Yamasaki M, Yoshikawa I, Otsuki M. Argon plasma coagulation therapy for a hemorrhagic radiationinduced gastritis in patient with pancreatic cancer. Intern Med. 2007; 46:975–7.

6. Furukawa K, Ho N, Kuroda K, Ikarashi K, Hata K, Tukioka S. A case of radiation hemorrhagic gastritis successfully treated by endoscopic argon plasma coagulation. Endosc Forum Dig Dis. 2003; 19:40–4.
7. Wada S, Tamada K, Tomiyama T, Yamamoto H, Nakazawa K, Sugano K. Endoscopic hemostasis for radiation-induced gastritis using argon plasma coagulation. J Gastroenterol Hepatol. 2003; 18:1215–8.

8. Staiano T, Grassia R, Iiritano E, Bianchi G, Dizioli P, Buffoli F. Treatment of radiation-induced hemorrhagic gastritis with endoscopic band ligation. Gastrointest Endosc. 2010; 72:452–3.

9. Flobert C, Cellier C, Landi B, Berger A, Durdux C, Palazzo L, et al. Severe hemorrhagic gastritis of radiation origin. Gastroenterol Clin Biol. 1998; 22:232–4.
10. Yeung YP, Ho CM, Wong KH, Lam KH, Cheung WY, Wong AW, et al. Surgical treatment of recalcitrant radiation-induced gastric erosions. Head Neck. 2000; 22:303–6.

11. Inaba K, Sakurai Y, Furuta S, Sunagawa R, Tonomura S, Nakamura Y, et al. Hemorrhagic radiation gastritis after chemoradiation therapy for peritoneal lymph node metastasis of esophageal carcinoma successfully treated with repeated intraarterial steroid infusions. Esophagus. 2007; 4:67–72.

12. Zhang L, Xie XY, Wang Y, Wang YH, Chen Y, Ren ZG. Treatment of radiation-induced hemorrhagic gastritis with prednisolone: a case report. World J Gastroenterol. 2012; 18:7402–4.

13. Toda K, Shiratori Y, Yasuda M, Enya M, Uematsu T, Shimazaki M, et al. Therapeutic effect of intraarterial prednisolone injection in severe intestinal Behcet's disease. J Gastroenterol. 2002; 37:844–8.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download