Abstract
T-lymphoblastic lymphoma (T-LBL) is a rare form of aggressive non-Hodgkin’s lymphoma. The standard approach for management of T-LBL involves intensive multiagent chemotherapy regimens for induction and consolidation phases with central nervous system prophylaxis and a maintenance phase lasting 12-18 months. We report on a case of long-term survival after one cycle of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) and high-dose methotrexate. A 30-year-old woman diagnosed with T-LBL with a large mediastinal mass underwent one cycle of hyper-CVAD. Four days after the start of treatment, the mediastinal mass was markedly reduced. Treatment continued with one cycle of consolidation chemotherapy, comprising high-dose methotrexate and high-dose cytarabine. The patient then refused all further chemotherapeutic treatment. Seven years have passed without relapse.
Lymphoblastic lymphoma (LBL) is an aggressive form of non-Hodgkin’s lymphoma (NHL), comprising approximately 2% of all adult NHL cases [1]. LBL generally shows a high response rate to initial chemotherapy; however, relapse is common and overall survival is poor with treatment using conventional lymphoma regimens [2]. Recently developed chemotherapy regimens include intensive remission induction chemotherapy, central nervous system (CNS) prophylaxis, consolidation chemotherapy, and subsequent maintenance therapy for 12-18 months [3]. Here, we report on a case in which complete remission (CR) was achieved and the patient survived more than seven years after only one cycle of induction chemotherapy and consolidation therapy.
A 30-year-old female presented to our hospital with dyspnea and chest discomfort for three weeks. The patient’s past medical history was unremarkable. No fever or night sweats were reported. Findings on physical examination were as follows: body temperature, 36.8°C; blood pressure, 140/60 mm Hg; and pulse rate, 112 beats per minute. Heart sounds were normal, with no murmur. Decreased Lung sounds were detected in the right lower lung fields. On palpitation, bilateral supraclavicular lymph nodes were found to be approximately 1 cm in size, non-tender, and fixed. Results of laboratory tests were as follows: white cell count, 6,640 /μL; hemoglobin, 15.3 g/dL; and platelets, 450,000 /μL. Blood chemistry findings were as follows: aspartate aminotransferase, 34 IU/L; alanine aminotransferase, 23 IU/L. Lactic dehydrogenase (LDH)-level increased to 1,210 IU/L. Computed tomography (CT) of the chest showed a large mediastinal mass, right pleural effusion, pericardial effusion, and enlarged lymph nodes in the supraclavicular area, internal mammary area, and both axillae (Fig. 1A). Abdominal CT scan showed enlarged lymph nodes in the left para-aortic and retrocaval areas (Fig. 1B).
An excisional biopsy was performed on the left axillary lymph node. Hematoxylin and eosin staining showed immature nuclei with fine chromatin, and convoluted nuclear contours in most tumor cells (Fig. 2A). Based on immunohistochemical staining, tumor cells were positive for CD3 (Fig. 2B), CD5 (Fig. 2C), and CD99 (Fig. 2D) but negative for TdT and CD30. The proliferation index (Ki-67) was 90%. Diagnosis of precursor T-LBL was based on these findings. No evidence of bone marrow involvement was found, and no tumor cells were detected in cerebrospinal fluid.
One cycle of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) chemotherapy consisting of intravenous hyperfractionated cyclophosphamide (300 mg/m2 every 12 hours on days 1-3), vincristine (2 mg/m2 on day 4), doxorubicin (50 mg/m2 on day 3), and dexamethasone (40 mg on days 1-4) was administered. For CNS prophylaxis, intrathecal methotrexate (12 mg on day 2) was administered. Four days after initiation of remission induction chemotherapy, chest radiography showed that the mediastinal mass had almost disappeared (Fig. 3). One cycle of high-dose methotrexate (1 g/m2 on day 1) and cytarabine (3 g/m2 every 12 hours on days 2 and 3) was then administered. Three additional cycles of hyper-CVAD, high-dose methotrexate, and cytarabine were scheduled, but the patient refused all further chemotherapeutic treatment.
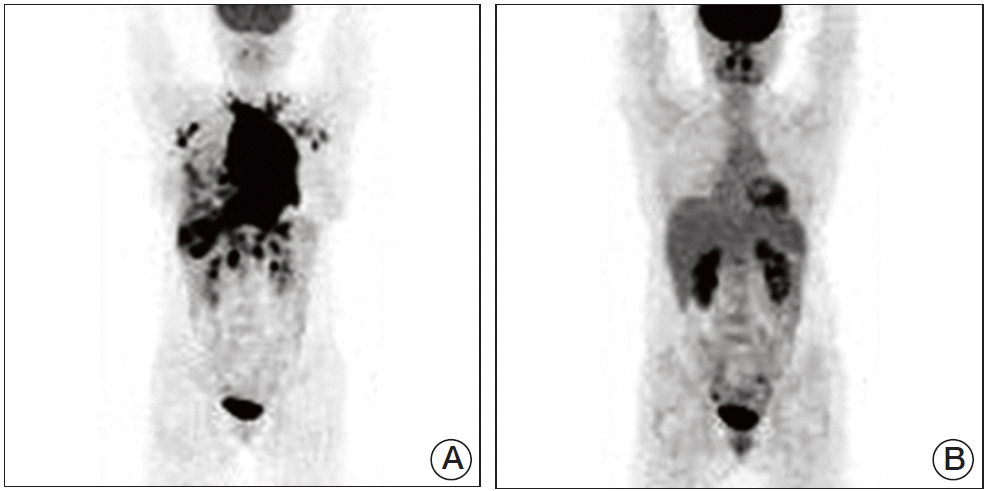
Follow-up was discontinued for five months. Seven months after hyper-CVAD chemotherapy, chest radiography showed no mediastinal mass and positron emission tomography (PET)-CT scans showed no hypermetabolic lesion (Fig. 4). The patient gave birth two years after undergoing chemotherapy. At the time of preparation of this case report, the patient had been in CR for 87 months.
T-LBL accounts for 90% of cases of LBL. T-LBL is typically seen in adolescents and young adults, predominantly in males (male:female, 3:1 ratio). T-LBL presents as a mediastinal mass in 60-70% of cases, and is often accompanied by shortness of breath due either to compression of the superior vena cava or pericardial or pleural effusion [2]. Bone marrow involvement is frequent, and a 5-10% incidence of CNS involvement at presentation has been reported. The pathologic characteristics of LBL at the morphologic, phenotypic, and genetic levels are similar to those of acute lymphoblastic leukemia (ALL). The World Health Organization classification of lymphoid neoplasms has unified these entities as precursor T-cell or B-cell lymphoblastic leukemia/lymphoma. However, some recent studies have suggested different molecular profiles for T-LBL and T-ALL [4-6], and the response of T-LBL to therapy appears to differ from that of T-ALL [4,7]. Therefore, clinical distinction of the two diseases is valid and specific research on LBL treatment has beenconducted.
Conventional NHL treatment protocols yielded low rates of CR, disease free survival, and high relapse rates. Although intensive protocols for aggressive NHL have increased the CR rate, overall survival remains poor [8]. Improvements in long-term outcome have been achieved with ALL type regimens for LBL, and in multiple series, CR rates of 50-91% and disease free survival rates of 45-67% have been reported [9-11]. No optimal standard induction therapy has emerged; however, many chemotherapy regimens with similarities to ALL regimens have recently been developed.
The hyper-CVAD regimen used in the case reported here normally entails eight induction-consolidation courses alternating hyper-CVAD with high-dose methotrexate and cytarabine. Maintenance therapy included 6-mercaoptopurine, methotrexate, vincristine, and prednisone (POMP) for 24 months. CR was 91%, and 3-year progression-free and overall survival was 66% and 70%, respectively [9]. In the current case, only one cycle of hyper-CVAD and one cycle of high-dose methotrexate, and cytarabine were administered. Four days after the initiation of remission induction chemotherapy, the mediastinal mass had almost disappeared. After one cycle of consolidation chemotherapy, the patient was temporarily lost to follow-up because of treatment refusal. Follow-up PET-CT scan performed seven months after chemotherapy showed CR, which has been maintained.
Temporary spontaneous remission of T-LBL has been previously reported [12]. However, no report on CR and long-term survival after one cycle of chemotherapy has been published. Unlike T-ALL, no reliable prognostic factors have been identified for T-LBL. The prognosis of B-LBL is known to be better than that for T-LBL [8]. In an MD Anderson Cancer Center study, only CNS involvement at diagnosis showed significant association with poor outcome [9]. In a childhood ALL study, no reliable prognostic factors were identified [13]. Coleman et al. [14] classified patients into high and low risk groups according to the Ann Arbor stage, bone marrow involvement, and serum LDH. Rates of freedom from relapse in the low risk and high risk groups at five years were 94% and 19%, respectively. However, in the German Multicenter Study Group for adult ALL (GMALL) series, no significant difference was observed between the two groups [15]. The value of minimal residual disease as a prognostic factor for LBL should be determined in future studies. Sweetenham [3] identified the intensity of induction therapy as essential to long-term survival. This factor was found to have a greater impact on outcome than that by consolidation or maintenance therapy. Early intrathecal therapy and CNS prophylaxis were effective for treatment of T-LBL in our patient. Rapid and deep response may facilitate long-term survival at an early stage of remission induction chemotherapy. LBL is treated with a chemotherapy regimen similar to that used in patients with ALL. However, due to rarity of the disease and no reliable prognostic factor, no report on reduction of chemotherapy cycle or omission of maintenance therapy has been published.
In conclusion, we report on a case of a patient with T-LBL who achieved long-term CR with no post-induction chemotherapy.
References
1. A clinical evaluation of the International Lymphoma Study
Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997; 89:3909–18.
3. Sweetenham JW. Treatment of lymphoblastic lymphoma in adults. Oncology (Williston Park). 2009; 23:1015–20.
4. Hoelzer D, Gokbuget N. T-cell lymphoblastic lymphoma and T-cell acute lymphoblastic leukemia: a separate entity? Clin Lymphoma Myeloma. 2009; 9 Suppl 3:S214–21.

5. Raetz EA, Perkins SL, Bhojwani D, Smock K, Philip M, Carroll WL, et al. Gene expression profiling reveals intrinsic differences between T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Pediatr Blood Cancer. 2006; 47:130–40.

6. Uyttebroeck A, Suciu S, Laureys G, Robert A, Pacquement H, Ferster A, et al. Treatment of childhood T-cell lymphoblastic lymphoma according to the strategy for acute lymphoblastic leukaemia, without radiotherapy: long term results of the EORTC CLG 58881 trial. Eur J Cancer. 2008; 44:840–6.

7. Jabbour E, Koscielny S, Sebban C, Peslin N, Patte C, Gargi T, et al. High survival rate with the LMT-89 regimen in lymphoblastic lymphoma (LL), but not in T-cell acute lymphoblastic leukemia (T-ALL). Leukemia. 2006; 20:814–9.

8. Cortelazzo S, Ponzoni M, Ferreri AJ, Hoelzer D. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2011; 79:330–43.

9. Thomas DA, O'Brien S, Cortes J, Giles FJ, Faderl S, Verstovsek S, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004; 104:1624–30.

10. Hoelzer D, Gokbuget N. Treatment of lymphoblastic lymphoma in adults. Best Pract Res Clin Haematol. 2002; 15:713–28.

11. Bouabdallah R, Xerri L, Bardou VJ, Stoppa AM, Blaise D, Sainty D, et al. Role of induction chemotherapy and bone marrow transplantation in adult lymphoblastic lymphoma: a report on 62 patients from a single center. Ann Oncol. 1998; 9:619–25.

12. Ceesay MM, Vadher B, Tinwell B, Goderya R, Sawicka E. Spontaneous remission of T lymphoblastic lymphoma. J Clin Pathol. 2008; 61:955–7.

13. Reiter A, Schrappe M, Ludwig WD, Tiemann M, Parwaresch R, Zimmermann M, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood. 2000; 95:416–21.
Fig. 1.
Computed tomography (CT) at initial diagnosis. (A) Chest CT scan showed a large mediastinal mass, pleural effusion. (B) Abdominal CT scan showed left para-aortic lymphadenopathy.
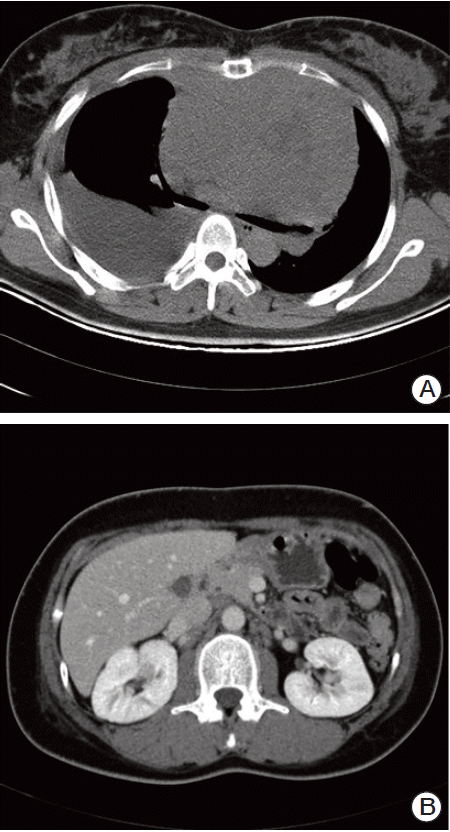
Fig. 2.
Microscopic findings of the precursor T-cell lymphoblastic lymphoma from the axillary lymph node. (A) Most tumor cells show immature nuclei with fine chromatin, convoluted nuclear contours, and frequent mitotic figures (H&E staining, ×1,000). On immunohistochemical staining, tumor cells were positive for CD3 (B) CD5 (C), and CD99 (D) (B-D, ×400).
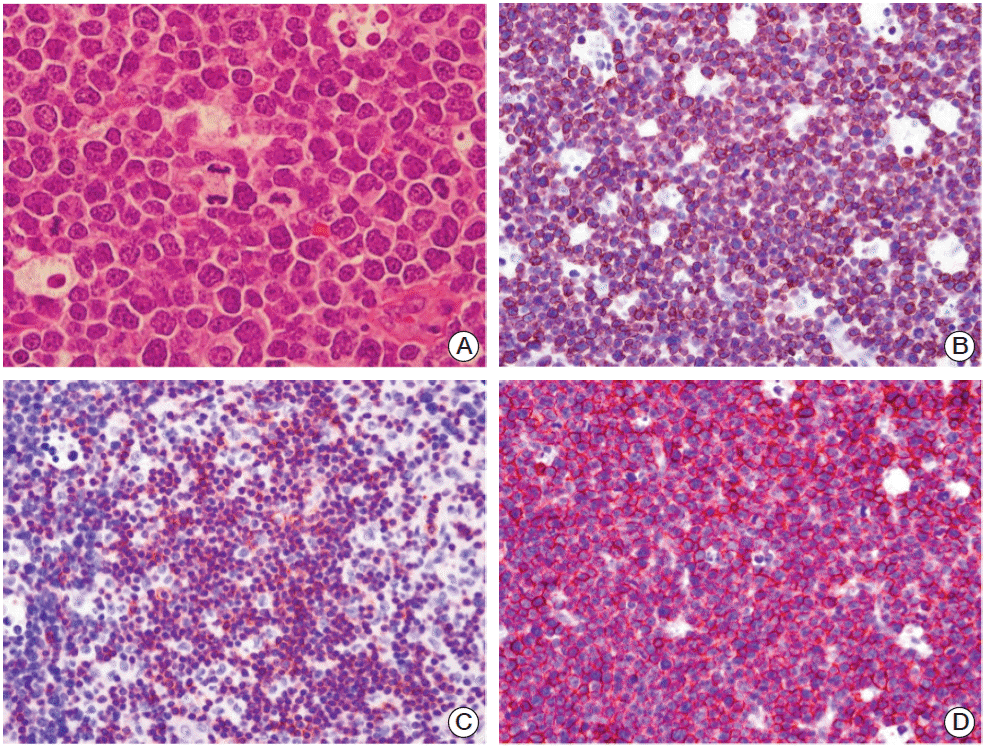
Fig. 3.
Comparison of chest X-ray. (A) Before treatment of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), on April 3, 2006. (B) Chest X-ray showed that the mediastinal mass had decreased after performance of hyper-CAVD, on April 7, 2006.
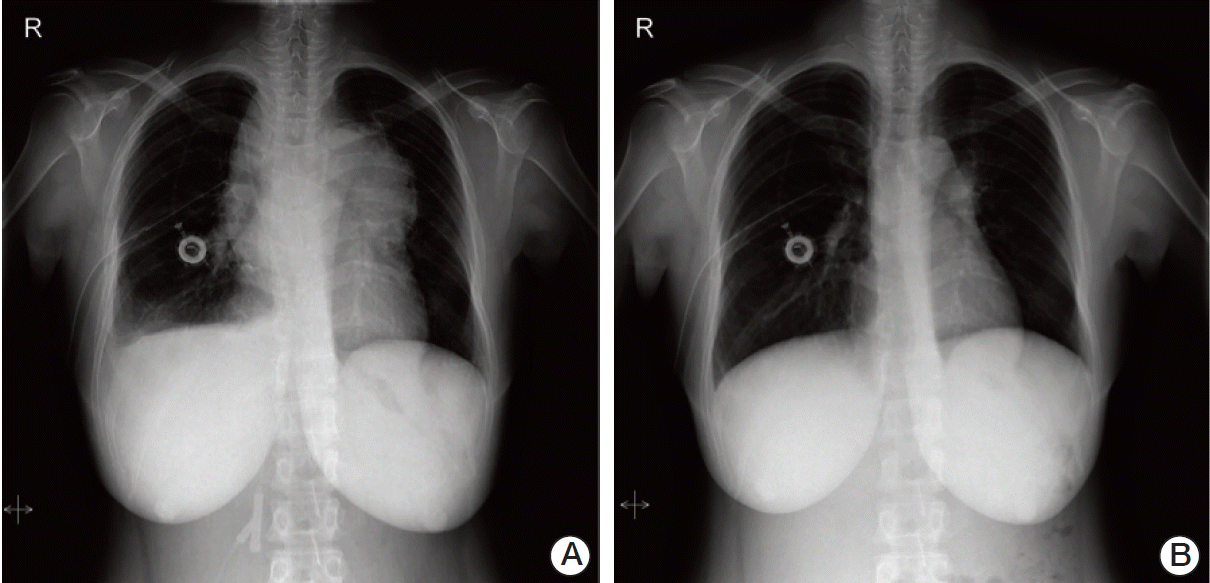
Fig. 4.
Comparison of position emission tomography-computed tomography (PET-CT), before and after treatment. (A) In March 2006, PET-CT showed the hypermetabolic mediastinal mass with multiple lymphadenopathy and pleural invasion. (B) In November 2006, after chemotherapy, PET-CT showed no hypermetabolic lesion.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download