Introduction
Materials and Methods
1. Cell culture
2. Cell viability assay
3. VEGF gene expression analysis by reverse transcriptase-polymerase chain reaction (PCR)
4. Western blot analysis
5. Detection of NF-κB activity
6. Immunofluorescence
7. Apoptosis assay
8. Measurement of caspase-3 activity
9. Statistical analysis
Results
1. Celecoxib increased paclitaxel-induced growth inhibition of human ovarian cancer cell line OVCAR-3
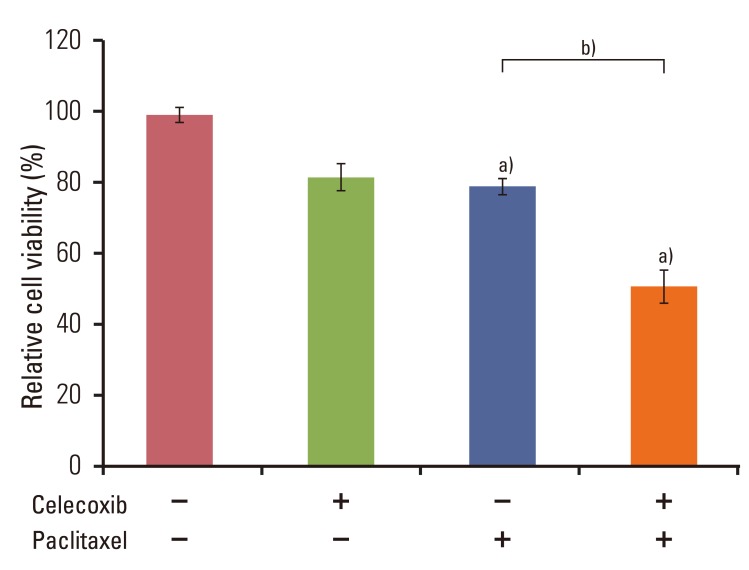 | Fig. 1Combined use of celecoxib and paclitaxel reduces cell viability of the human ovarian cancer cell line OVCAR-3. Cells were pretreated of celecoxib (10 µM) for 1 hour before addition of paclitaxel (20 µM) for 6 hours. Cell viability was measured using the Cell Counting Kit-8 solution as described in the Materials and Methods section of this paper. Results are representative of three experiments. Data in the bar graph represent mean±SD. a)p<0.05 vs. control, b)p<0.05 vs. paclitaxel. |
2. Celecoxib enhanced paclitaxel-induced apoptosis in OVCAR-3 cells
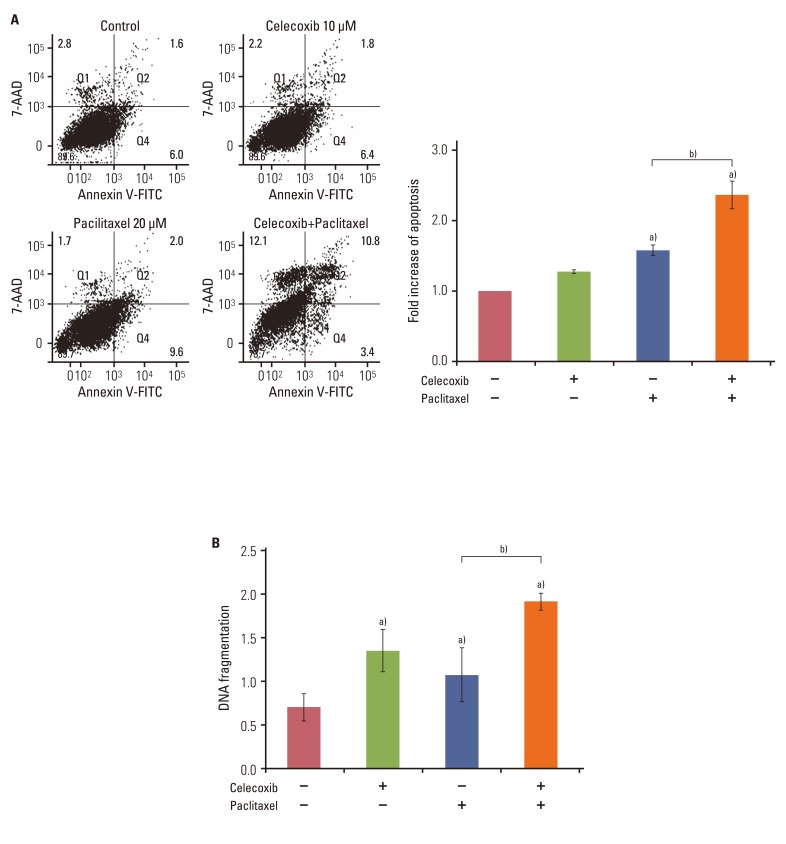 | Fig. 2Celecoxib promotes paclitaxel-induced apoptosis in OVCAR-3 cells. Cells (1×105 cells/well) were pretreated with or without celecoxib (10 µM) for 1 hour before addition of paclitaxel (20 µM). (A) Apoptosis was assessed by Annexin V-FITC/7-aminoactinomycin D (7-AAD) staining after treating OVCAR-3 cells with paclitaxel for 48 hours, followed by fluorescence activated cell sorting analysis. Gate settings distinguish between living (bottom left), necrotic (top left), early apoptotic (bottom right), and late apoptotic (top right) cells. In the right panel, the levels of apoptosis are presented as the fold increase relative to the apoptosis of untreated OVCAR-3 cells. (B) Apoptosis was measured by cellular DNA fragmentation enzyme-linked immunosorbent assay. Results are representative of three experiments. Data are presented as mean±SD. a)p<0.05 vs. control, b)p<0.05 vs. paclitaxel treated group. |
3. Celecoxib increased paclitaxel-induced caspase a activation and PARP cleavage
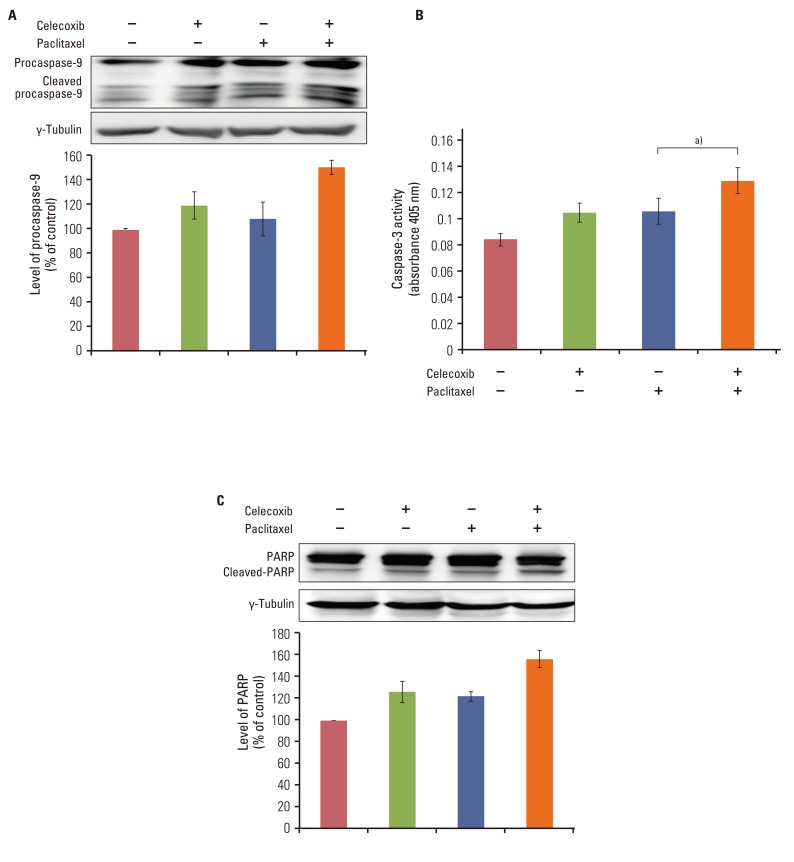 | Fig. 3Celecoxib enhances paclitaxel-induced caspase activation and poly ADP-ribose polymerase (PARP) cleavage. (A) Cell lysates from cells incubated in the presence or absence of celecoxib (10 µM) for 1 hour following stimulation with paclitaxel (20 µM) for 24 hours were subjected to western blotting with anti-procaspase-9 antibody. (B) Caspase-3 activity was measured by colorimetric assay. Data are presented as mean±SD. a)p<0.05 vs. paclitaxel treated group. (C) Cells were incubated with or without celecoxib (10 µM) for 1 hour before addition of paclitaxel (20 µM). After 24 hours, cells were harvested and cleavage of PARP was analyzed by western blotting using anti-PARP antibody. The band intensities were quantitated. |
4. Celecoxib inhibited paclitaxel-induced NF-κB activation and VEGF mRNA expression
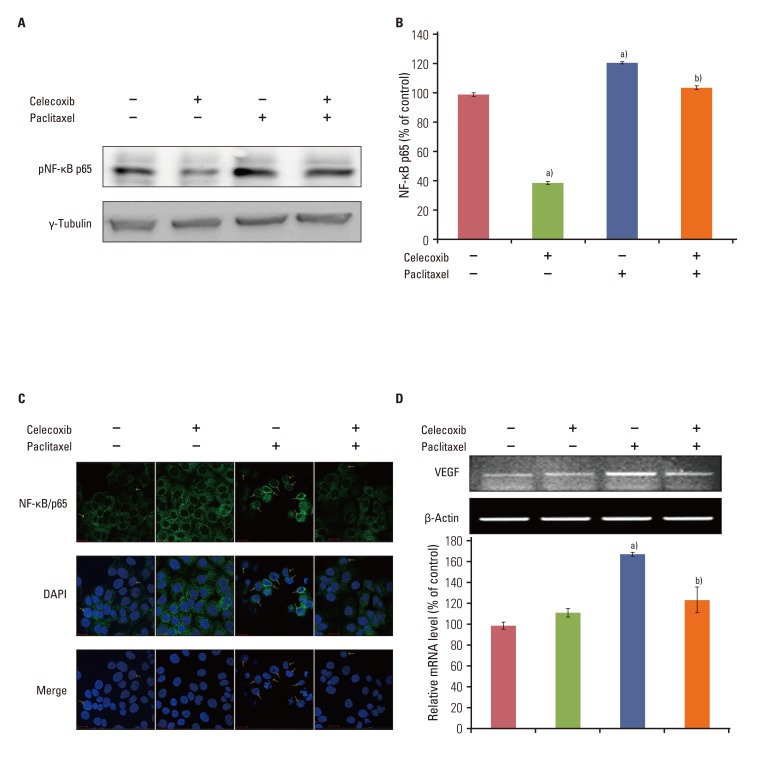 | Fig. 4Celecoxib reduces the activation of nuclear factor-κB (NF-κB) and vascular endothelial growth factor (VEGF) mRNA induced by paclitaxel. OVCAR-3 cells were pretreated of celecoxib (10 µM) for 1 hour before addition of paclitaxel (20 µM) for 6 hours. The nuclear extract was collected. (A) Western blot analysis of pNF-κB p65. (B) The levels of active NF-κB family members were determined using the Trans AM NF-κB kit according to the manufacturer's instructions and reported as an absorbance level of the colorimetric substrate. (C) NF-κB/p65 localization was visualized using an anti-NF-κB/p65 primary antibody followed by an Alexa Fluor 488-conjugated detection antibody. (D) RNA was purified with Trizol as described in the Materials and Methods section of this paper. One microgram of total RNAs was subjected to reverse transcriptase-polymerase chain reaction for detection of VEGF mRNA expression. Results are representative of three experiments. Data in the bar graph represent mean±SD. a)p<0.05 vs. control, b)p<0.05 vs. paclitaxel. |
5. Celecoxib decreased paclitaxel-induced Akt activation in OVCAR-3 cells
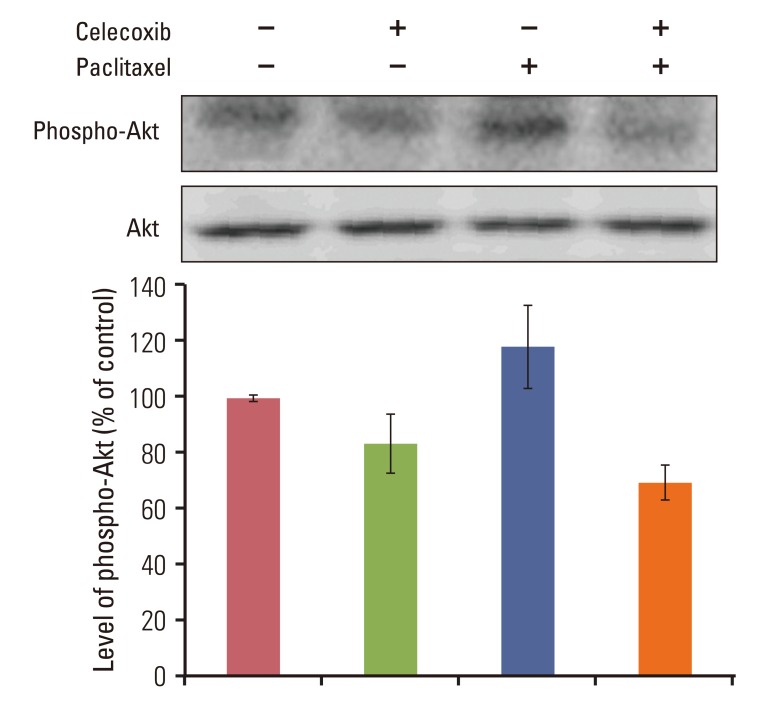 | Fig. 5Celecoxib reduces paclitaxel-induced Akt phosphorylation in OVCAR-3 cells. Cells were incubated with or without celecoxib (10 µM) for 1 hour before addition of paclitaxel (20 µM). After 24 hours, cells were harvested and Akt activation was analyzed by western blotting using anti-phospho-Akt antibody. The band intensities were quantitated. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download