Abstract
Intravascular large B-cell lymphoma (IVLBCL) is a rare subtype of non-Hodgkin lymphoma. It usually presents with nonspecific symptoms, such as fever, rather than with overt lymphadenopathy. Reports of hypercalcemia, as the initial presentation of IVLBCL, are limited in the literature, despite it being a well-known complication of various solid cancers. We present a 68-year-old male with severe hypercalcemia and increased levels of serum parathyroid hormone-related protein. He was diagnosed with IVLBCL, involving the bone marrow and spleen, and was successfully treated with rituximab-containing chemotherapy. A few previous case reports have shown hypercalcemia in patients with IVLBCL. Much like our case, previous cases with hypercalcemia had advanced diseases, including bone marrow invasion. Although it was an extremely rare manifestation of IVLBCL, we suggest that IVLBCL should be a part of the differential diagnosis in patients with unexplained hypercalcemia. Therefore, an active work-up might be recommended, including positron emission tomography/ computed tomography scan and bone marrow examination, which may be useful for early diagnosis.
Intravascular large B-cell lymphoma (IVLBCL) is an extremely rare subtype of non-Hodgkin lymphoma, characterized by the presence of CD20-positive large B-cell lymphoma cells in the lumina of the small blood vessels [1]. The diagnosis of IVLBCL is often delayed, as it mainly presents with nonspecific symptoms, such as fever and weakness, and peripheral lymph node enlargements are rarely observed [2,3]. Thus, IVLBCL can be initially misdiagnosed as an infectious or autoimmune disorder. Due to its clinical obscurity and extremely low incidence rate, a timely diagnosis of IVLBCL is difficult, resulting in treatment delays that may eventually lead to poor prognosis. However, as clinical and laboratory information regarding IVLBCL has accumulated in the literature, including case series, the understanding of this rare disease has improved. With such understanding, development of diagnostic and treatment approaches was possible [1,4]. Nevertheless, early suspicion of IVLBCL is still one of the most helpful factors in accurate diagnosis and better treatment outcome. Recently, we experienced a case of IVLBCL presented with altered mental state and severe hypercalcemia. Although hypercalcemia occurs in up to 20% to 30% of cancer patients [5], it is an unusual manifestation in patients with IVLBCL, and only a few reports have been published regarding the association between hypercalcemia and IVLBCL [6-10]. We, therefore, report this case with a review of the literature, and underscore IVLBCL as a possible cause of hypercalcemia.
A 68-year-old male patient was referred to our hospital due to a rapidly deteriorating mental state with persistent fever. For two months, he had often been feeling febrile, easily fatigued, and lethargic. As a result, he had lost more than 7 kg during this period. However, his physical findings did not show any evidence of neurologic signs that may explain his altered mentality or any abnormal lesions on his body, such as lymph node enlargement, a palpable liver or spleen, or a skin rash. His complete blood cell count showed only mild leucopenia (white blood cell count 2,560/μL, absolute neutrophil count 1,430/μL, hemoglobin 13.5 g/dL, and platelets 108,000/μL). Other laboratory results, including coagulation markers, liver function tests, electrolytes, and urine analysis, were all within normal ranges. A brain computed tomography (CT) scan was urgently performed due to his altered mental state, however, there was no evidence of vascular accidents, such as intracranial hemorrhage. Although C-reactive protein levels were increased (4.48 mg/dL; normal, 0 to 0.3 mg/dL), pro-calcitonin levels (0.26 ng/mL) were within the normal range (0 to 0.5 ng/mL). Blood microbial culture results showed no pathogen growth. However, serum calcium was extremely elevated to 20.6 mg/dL (normal range, 8.4 to 10.2 mg/dL) with elevated serum lactate dehydrogenase (LDH) (2,668 IU/L; normal, 240 to 480 IU/L) and slightly impaired renal function (blood urea nitrogen 43.3 mg/dL, creatinine 1.39 mg/dL). Cerebrospinal fluid analysis via lumbar puncture did not show any abnormal findings. Besides mild splenomegaly, chest and abdomen CT scan did not show any abnormal findings, such as lymph node enlargement or abnormal mass formation. Furthermore, no osteolytic lesions were found in the chest wall, pelvis, or vertebrae. While the patient was managed with supportive care for hypercalcemia, including saline hydration and administration of loop diuretics and calcitonin, a positron emission tomography (PET)/CT with 18F-fluorodeoxyglucose (FDG) was performed to exclude the possibility of hidden malignancy. A strong diffuse uptake of FDG was noted in the bones and spleen from a PET/CT scan (Fig. 1A). A subsequent bone marrow aspiration showed a presence of large atypical lymphoid cells. A peripheral blood smear showed hemophagocytosis (Fig. 2A and B), and the intrasinusoidal large atypical cells stained positive for CD20 (Fig. 2C). As a result, the patient was diagnosed with IVLBCL and treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). Over the course of R-CHOP chemotherapy, hypercalcemia was corrected and returning his mental state back to a normal, alert state. The initial blood tests, which were performed to explore the cause of severe hypercalcemia before the diagnosis of IVLBCL, could not explain the cause of hypercalcemia. His serum intact parathyroid hormone (iPTH) level was decreased (0.8 pg/mL; normal, 11 to 62 pg/mL) and his 1,25-dihydroxycholecalciferol [1,25-(OH)2D3] level was within the normal range (22.09 pg/mL; normal, 19.6 to 54.3 pg/mL). However, serum PTH-related protein (PTHrP) levels were increased (2.7 pmol/L) compared to the normal range (0 to 1.1 pmol/L). Therefore, his severe hypercalcemia might be associated with the increased level of PTHrP. This finding might suggest that there exist the possibility of PTHrP production from lymphoma cells. A follow-up PET/CT scan after the sixth cycle of R-CHOP showed a complete disappearance of the lesions, which was confirmed by bone marrow aspiration and biopsy (Fig. 1B). At present, he is alive and in a state of complete response (survival duration, 16 months), showing normal levels of serum calcium.
When an elderly patient presents with altered mental state and fever, infectious disorders and intracranial vascular disorders are usually suspected first. However, in this case, the results of blood tests and imaging studies were not consistent with these disorders. Instead, severe hypercalcemia and high LDH levels were the only abnormal findings that might have been associated with his clinical manifestations. His hypercalcemia was not explained by hyperparathyroidism, hyperthyroidism, sarcoidosis, nephrogenic diabetes insipidus, or medications, such as lithium, calcitriol, vitamin D, or thiazides [5,11]. Although myeloma-related osteolysis or metastatic bone tumors, such as prostate cancer, were also considered in this case as a cause of hypercalcemia; however, laboratory tests, such as protein electrophoresis and prostatespecific antigen, as well as a chest and abdomen CT scan did not provide any evidence to confirm these diseases. However, PET/CT scan and bone marrow study demonstrated the presence of B-cell lymphoma, leading to the final diagnosis of IVLBCL.
Hypercalcemia can occur in cancer patients via four mechanisms. First, PTHrP, secreted from malignant tumor cells, can induce bone resorption and renal retention of calcium. Second, osteolysis can increase the serum calcium levels due to an increase in osteoclastic bone resorption in areas surrounding the malignant cells. Third, secretion of the active form of vitamin D [1,25(OH)2D3] from cancer cells can enhance osteoclastic bone resorption and intestinal absorption of calcium. Lastly, ectopic secretion of authentic PTH is also possible, but rare. In B-cell lymphoma, hypercalcemia is reported in only 7-8% of cases, whereas hypercalcemia develops in approximately 30% of cases of myeloma, and 60% of cases of adult T-cell leukemia/lymphoma. Furthermore, the main mechanism of hypercalcemia in B-cell lymphoma is the uncontrolled production of 1,25-(OH)2D3 from tumor cells [5,12,13]. However, our case showed an elevation of PTHrP, while iPTH levels were low and 1,25-dihydroxycholecalciferol [1,25-(OH)2D3] levels were within the normal range. Although we could not demonstrate the expression of PTHrP in tumor cells by immunohistochemitry, ectopic production of PTHrP from tumor cells of IVLBCL might be a possible cause of hypercalcemia observed in this case.
Up until now, only five cases of IVLBCL that show hypercalcemia, including our case, have been reported. An elevation of serum PTHrP levels was documented in all cases except one (Table 1). Among the four cases with PTHrP-related hypercalcemia, three cases were from Asian countries, and all cases showed bone marrow involvement. Considering that the Asian variant of IVLBCL frequently shows bone marrow involvement with hemophagocytosis, the occurrence of this PTHrP-related hypercalcemia in IVLBCL might be associated with the Asian variant, which is predominantly characterized by neurologic symptoms and skin lesions [1-3]. However, the number of reported cases of IVLBCL that present with hypercalcemia is too small; therefore, future studies should be warranted to explore the association between hypercalcemia with characteristics of Asian variant, such as bone marrow invasion, and hemophagocytosis. In addition, the majority of these cases occurred in elderly patients, including our case. This suggests that elderly patients with IVLBCL might be more susceptible to hypercalcemia. Although the outcomes of the previously reported IVLBCL cases with hypercalcemia were poor, our patient was successfully treated with R-CHOP. The overall survival has markedly improved since rituximab was added to the conventional CHOP and CHOP-like chemotherapy [14]. Hence, in consideration to the superior efficacy of R-CHOP over previous treatment modalities, an early suspicion of IVLBCL may be critical for IVLBCL patients presented with hypercalcemia.
In conclusion, we presented a case of IVLBCL with severe hypercalcemia and elevated levels of serum PTHrP. A comparison with previously reported cases showed a tendency of occurrence of hypercalcemia in elderly patients with bone marrow involvement. Therefore, we suggest that IVLBCL should be included in the differential diagnosis of elderly patients with unexplained hypercalcemia, and an active work-up, including PET/CT scan and a bone marrow exam, may be useful in achieving an early diagnosis.
References
1. Ponzoni M, Ferreri AJ, Campo E, Facchetti F, Mazzucchelli L, Yoshino T, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007; 25:3168–73.

2. Murase T, Nakamura S, Kawauchi K, Matsuzaki H, Sakai C, Inaba T, et al. An Asian variant of intravascular large B-cell lymphoma: clinical, pathological and cytogenetic approaches to diffuse large B-cell lymphoma associated with haemophagocytic syndrome. Br J Haematol. 2000; 111:826–34.

3. Ferreri AJ, Campo E, Seymour JF, Willemze R, Ilariucci F, Ambrosetti A, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the 'cutaneous variant'. Br J Haematol. 2004; 127:173–83.
4. Masaki Y, Dong L, Nakajima A, Iwao H, Miki M, Kurose N, et al. Intravascular large B cell lymphoma: proposed of the strategy for early diagnosis and treatment of patients with rapid deteriorating condition. Int J Hematol. 2009; 89:600–10.

5. Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005; 352:373–9.
6. Yousem SA, Colby TV. Intravascular lymphomatosis presenting in the lung. Cancer. 1990; 65:349–53.

7. Knobel B, Sommer I, Petchenko P, Lev D, Okon E. Malignant humoral hypercalcemia associated with angiotropic large B cell lymphoma. Harefuah. 2001; 140:204–87. 204-6, 87.
8. Hanihara T, Takahashi T, Shimada T, Mizuguchi M, Yagishita S. Parathyroid hormone-related protein-associated hypercalcemia in probable intravascular lymphoma of B-cell type. Am J Hematol. 1996; 53:144–5.

9. Curtis JL, Warnock ML, Conrad DJ, Helfend LK, Boushey HA. Intravascular (angiotropic) large-cell lymphoma ('malignant angioendotheliomatosis') with small vessel pulmonary vascular obstruction and hypercalcemia. West J Med. 1991; 155:72–6.
10. Chinen Y, Nakao M, Sugitani-Yamamoto M, Kiyota M, Horiike S, Kuroda J, et al. Intravascular B-cell lymphoma with hypercalcemia as the initial presentation. Int J Hematol. 2011; 94:567–70.

12. Seymour JF, Gagel RF, Hagemeister FB, Dimopoulos MA, Cabanillas F. Calcitriol production in hypercalcemic and normocalcemic patients with non-Hodgkin lymphoma. Ann Intern Med. 1994; 121:633–40.

Fig. 1.
(A) Diffuse 18F-fluorodeoxyglucose (FDG) uptake can be seen in the bone marrow and spleen at the time of diagnosis. (B) Normalized FDG uptake can be seen in the same areas after the sixth cycle of chemotherapy.
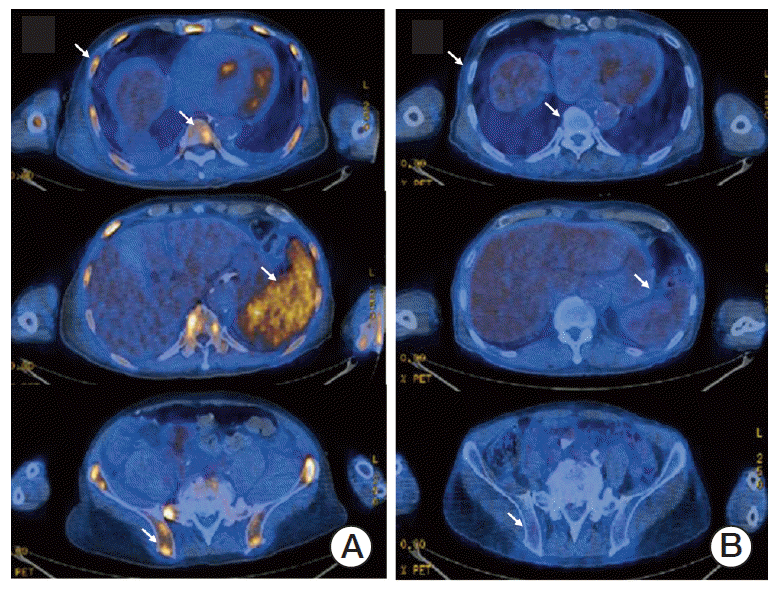
Fig. 2.
Histopathological findings (A and B, Wright Giemsa staining, ×400). (A) The bone marrow smear specimen shows increased numbers of histiocytes and hemophagocytosis. (B) The peripheral blood smear shows hemophagocytosis. Immunohistochemical staining for CD20 of the bone marrow biopsy specimen (C, ×400). (C) Lymphoma cells positive for CD20 invading sinusoids in the bone marrow.
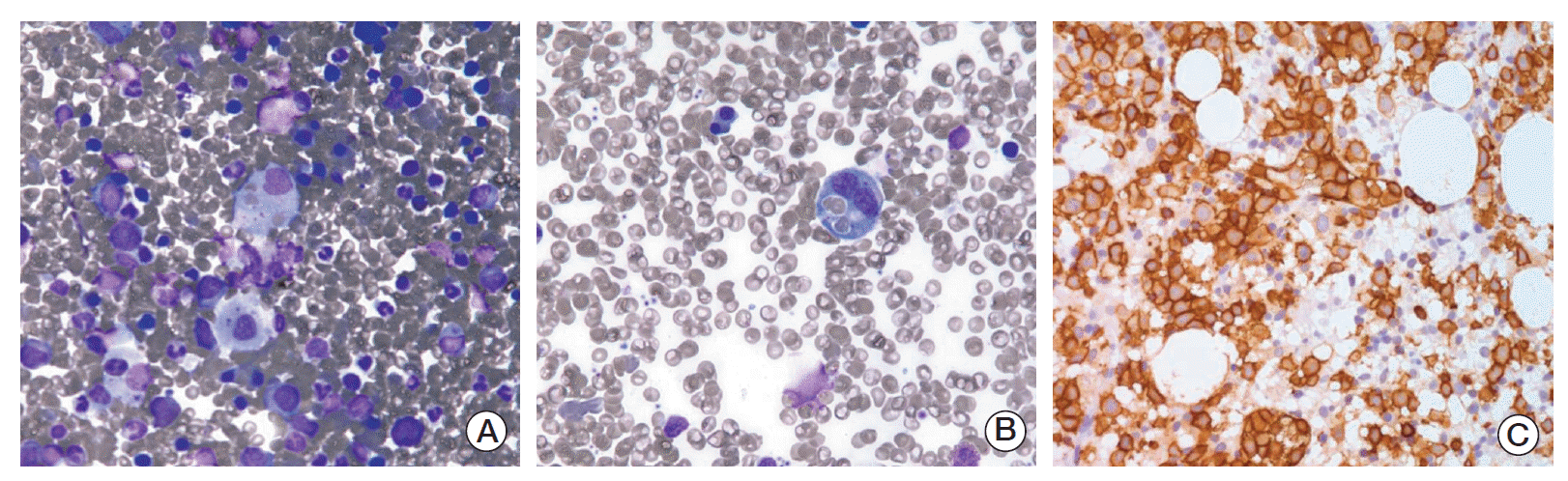
Table 1.
Clinical and laboratory data of reported patients with IVLBCL with hypercalcemia
| Age(yr)/Gender | Race | Ca(mg/dL) | PTHrP(normal range) | LDH (IU/L) | Involved sites | Initial symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 76/M | Guatemalana)[9] | 15.3 | UK | UK | Lung, BM, spleen, brain, liver, spinal cord, adrenal gland | Dyspnea, confusion, fever | Steroid | Early death |
| 64/F | Asian [8] | 13.1 | 151 pg/mL (13.8-55.3 pg/mL) | 1,750 | Brain, BM | Seizure, mental deterioration | CHOP | Early death |
| 52/M | Caucasian [7] | 18.6 | 8 pmol/L (< 0.8 pmol/L) | No data | Liver, BM | Not described | MACOP-B | Not described |
| 71/M | Asian [10] | 14.9 | 113 pmol/L (< 11 pmol/L) | 2,140 | Lung, kidney, BM | Lethargy, drowsy mentality, hypoxemia | R-CHOP with MTX | Relapse |
| 68/Mb) | Asian | 20.6 | 2.7 pmol/L (< 1.1 pmol/L) | 2,668 | BM | Stuporous mentality, lethargy, fever | R-CHOP | Alive in complete response |
IVLBCL, intravascular large B-cell lymphoma; PTHrP, parathyroid hormone-related protein; LDH, lactate dehydrogenase; M, male; BM, bone marrow; F, female; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; MACOP-B, methotrexate with leucovorin rescue, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; MTX, methotrexate.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download