Abstract
Purpose
The purpose of this study is to evaluate the outcome of low-dose whole brain radiotherapy (WBRT) with tumor bed boost after methotrexate-based chemotherapy in the management of primary central nervous system lymphoma (PCNSL).
Materials and Methods
We retrospectively analyzed 64 patients with pathologically proven PCNSL between 2000 and 2011. Methotrexate-based chemotherapy with a median of five cycles was followed by radiotherapy to the whole brain and to the initial tumor bed. The median dose to the whole brain and to the tumor bed was 27 Gy (range, 18 to 36 Gy) and 50.4 Gy (range, 45 to 54 Gy), respectively.
Results
With a median follow-up period of 27 months, 55 patients (85.9%) achieved complete response (CR). The 5-year overall survival (OS) and progression-free survival (PFS) rates were 52.6% and 39.3%, respectively. In univariate analysis, factors associated with OS were age, performance status, involvement of deep structure, and CR to sequential chemoradiotherapy (CRT). These variables remained as significant factors for OS in multivariate analysis. CR to sequential CRT was the only positive factor associated with PFS (p=0.009). Neurologic toxicity was more common in elderly patients older than 60 years (p=0.025).
Primary central nervous system lymphoma (PCNSL) is an aggressive form of non-Hodgkin lymphoma that develops in the brain, spinal cord, eyes, or leptomeninges without evidence of systemic involvement. High-dose methotrexate (HD-MTX) based chemotherapy followed by whole brain radiotherapy (WBRT) is the most common strategy for treatment of patients with PCNSL [1,2]. In comparison of historical data, the combination therapy improves survival [1-3].
However, the combination approach is associated with higher risk of severe neurologic toxicity (NT), particularly in elderly patients [1,3-5]. Various strategies for maintenance of treatment efficacy while reducing toxic effects have been explored. These include deferral of WBRT until tumor progression, hyperfractionated radiation schedule as in Radiation Therapy Oncology Group (RTOG) 02-27, substitution of WBRT with high-dose chemotherapy with autologous stem cell transplantation, or avoidance of WBRT in the elderly [5-7]. Studies on replacement of WBRT with conventional chemotherapy are still limited [8,9]. Findings of a phase 3 randomized clinical trial (G-PCNSL-SG-1) showed no significant difference in overall survival (OS) when WBRT was skipped after first-line chemotherapy, however, a disadvantage in progression-free survival (PFS) was proven regardless of chemotherapy response [10]. Therefore, it was thought that the benefit of WBRT in terms of PFS outweighed the increased risk of NT. Although diverse methods have been proposed, WBRT remains an essential part of treatment because no equivalent effect has been demonstrated from the other alternatives. Therefore, reducing radiation dose to the whole brain and using focal boost to the tumor bed could be a valid and practical way not only to minimize NT but also to maintain high tumor control rates.
In the current study, we evaluated the outcome of sequential chemoradiotherapy (CRT) using a low-dose WBRT with tumor bed boost for PCNSL. Findings of this study might provide some insights for use in clinical practice or in establishment of future clinical trials.
Our study analyzed immunocompetent PCNSL patients who were treated with CRT at Seoul National University Hospital from January 2000 to December 2011. Sixty four patients were eligible. After obtaining approval from the institutional review board, we performed a retrospective review of the medical records. After pathologic confirmation, all patients received HD-MTX based chemotherapy and then completed the planned dose of radiotherapy. Patients with involvement of extra-central nervous system (CNS) sites or with history of other malignancy were excluded.
A summary of the clinical characteristics is shown in Table 1. The 38 men and 26 women had a median age of 56 years (range, 23 to 75 years). Pathologic confirmation was performed in all patients by stereotactic biopsy in 54 patients (84.4%), gross total resection in seven (10.9%), and subtotal resection in three (4.7%). Forty-nine patients (76.6%) had multifocal lesions. In 41 patients (64.1%), the tumor had a deep structure involving the basal ganglia, corpus callosum, brainstem, or cerebellum. Twenty-five patients (39.1%) had a poor performance status (Eastern Cooperative Oncology Group [ECOG] scale ≥ 2) at the time of radiotherapy planning. Seven patients (10.9%) had involvement of malignant cells in the cerebrospinal fluid (CSF) at the time of diagnosis.
Upfront chemotherapy with the median of 5 cycles (range, 2 to 6) of the HD-MTX based regimen was used, which was similar to that used in the prior RTOG 93-10 study [1]. The dose of MTX was 2.5-3.5 g/m2 every two or three weeks. Methotrexate was infused over 6 hours on day 1 of each cycle with pre- and post-infusion hydration. Leucovorin rescue (20 mg orally every 6 hours for 12 doses) started around 24 hours after the start of MTX infusion. Vincristine 1.4 mg/m2 was administered once on day 1 of each cycle. Procarbazine 100 mg/m2 was administered orally for seven days during cycles 1, 3, and 5. Intrathecal MTX was administered to 15 patients (23.4%) with positive CSF cytology or suspicious leptomeningeal involvement. After completion of radiotherapy, two cycles of consolidation chemotherapy with high-dose cytarabine were used in 12 patients (18.8%). In addition, the combination of rituximab, MTX, procarbazine, and vincristine was used in five patients (7.8%) and concurrent CRT with temozolomide was used in three patients (4.7%).
Evaluation of response to upfront chemotherapy using contrast-enhanced magnetic resonance imaging (MRI) of the brain was performed in all patients before radiotherapy planning. Complete response (CR) was defined as complete disappearance of all enhancing or abnormal lesions on MRI (assisted with diffusion-weighted or perfusion images if available) and clearance of CSF or ocular involvement. Other responses were classified as non-CR.
Radiation therapy started three to six weeks after completion of upfront chemotherapy. All patients received WBRT followed by a boost to the tumor bed. The median dose to the whole brain and to the tumor bed was 27 Gy (range, 18 to 36 Gy) and 50.4 Gy (range, 45 to 54 Gy), respectively. Photons of 4 or 6 MV with conventional fractionation (1.8 Gy per day, five fractions per week) were used. A three-dimensional conformal radiotherapy was used in 56 patients (87.5%). Radiation doses were mainly based on the chemoresponse. Patients who achieved CR after upfront chemotherapy received the median dose of 27 Gy (range, 18 to 30.6 Gy) to the whole brain and median dose of 45 Gy (range, 45 to 54 Gy) to the tumor bed, whereas patients with non-CR received the median dose of 30.6 Gy (range, 19.8 to 36 Gy) to the whole brain and median dose of 50.4 Gy (range, 45 to 54 Gy) to the tumor bed. Whole spine radiotherapy was added in five patients whose initial CSF cytology was positive.
The median follow-up duration was 27 months (range, 5 to 151 months). The response evaluation was performed one month after completion of radiotherapy and was repeated every 3-4 months for two years, every six months for up to five years, and yearly thereafter. Because prospective neurologic function assessment was not performed, we performed retrospective analysis of NT in patients who achieved CR after radiotherapy. Treatment-related NT was defined as the occurrence of grade 2 or more neurologic symptoms (such as memory impairment, cognitive disturbance, or speech impairment) by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) ver. 3.0 [11]. Time to NT was calculated from completion of radiotherapy and was censored at the date of the last follow-up or at the time of intra-CNS failure to make certain that NT could clearly result from treatment induced toxicity.
OS was calculated from the date of pathologic confirmation. PFS was defined as the time from the date of pathologic confirmation to the date of relapse, progression, or the last date of follow-up. OS and PFS were determined using the Kaplan-Meier method and compared using the log-rank test. The Cox proportional-hazards model was used for multivariate analysis. Pearson’s chi-square test or Fisher’s exact test was used for comparison of the distribution of clinical parameters between two groups. SPSS release ver. 18.0.1 (SPSS Inc., Chicago, IL) was used for statistical analyses. Values of p < 0.05 indicated statistical significance.
CR was observed in 27 patients (42.2%) after upfront chemotherapy, in 43 patients (67.2%) one month after completion of radiotherapy, and in 55 patients (85.9%) during the follow-up period.
Five patients developed tumor progression and one had primary refractory PCNSL with progression during CRT. Twenty-six patients relapsed after achievement of complete remission. The relapse rate after CR was 47.3%. Fourteen patients relapsed in the brain alone, six patients in the brain and CSF space simultaneously, one patient in the CSF space alone, and two patients had isolated ocular recurrence. Two patients developed an isolated extra-CNS relapse (breast and retroperitoneum) and one patient had systemic lymph node relapse.
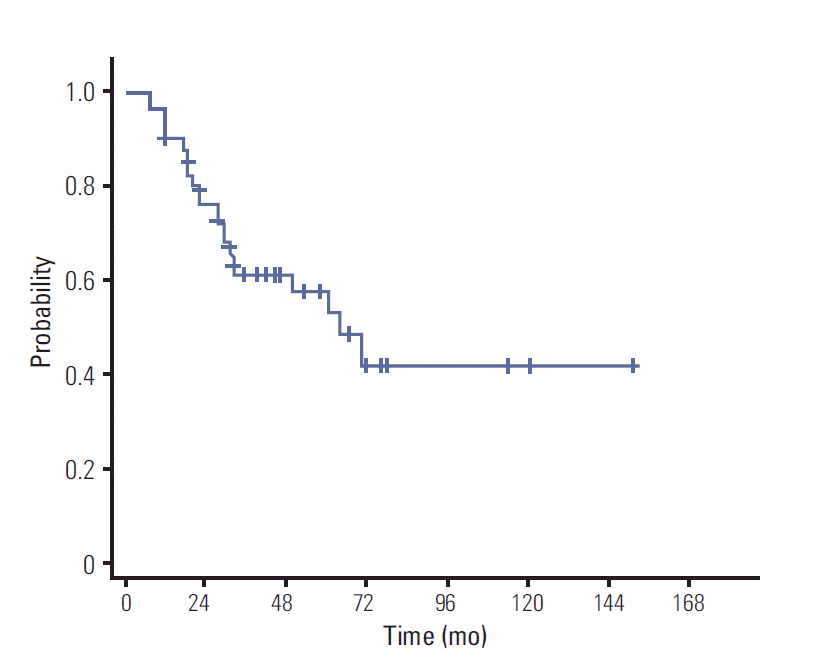
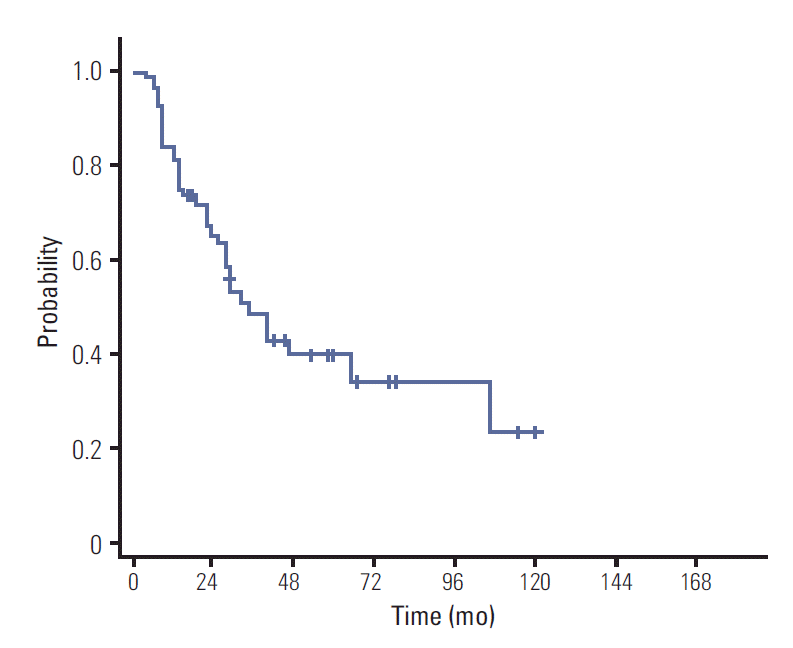
OS at 3- and 5-years was 59.8% and 52.6%, respectively. Three- and 5-year PFS was 47.9% and 39.3%, respectively (Figs. 1 and 2). Median OS and PFS were 63 months and 34 months, respectively. The results of univariate and multivariate analyses of OS and PFS are shown in Tables 2 and 3. In univariate analysis, four factors were associated with OS: age (p=0.022), performance status (p=0.012), involvement of deep structure (p=0.018), and CR to sequential CRT (p=0.019). These variables remained as significant factors in multivariate analysis (Table 3). In addition, CR to sequential CRT was the only factor showing association with better PFS (p=0.009) (Table 2).
Among 55 patients who achieved CR after CRT, 16 (29.1%) patients experienced NT. Memory impairment occurred in 12 patients (21.8%), speech impairment in eight (14.5%), and cognitive disturbance in seven (12.7%). In univariate analysis, age ≥ 60 was the only factor associated with freedom from NT (FFNT): 3-year FFNT rates were 71.5% in patients younger than 60 and 32% in those ≥ 60 (p=0.025). Other factors, including WBRT dose, co-administration of chemotherapy, or intrathecal chemotherapy did not show an association with FFNT.
Analysis of this single-institution experience suggests that low-dose WBRT with tumor bed boost after HD-MTX based chemotherapy is an effective method for management of PCNSL. Results of our study demonstrated a comparable OS with respect to previously reported studies using sequential CRT (Table 4) [1-3,12-15]. In addition, an important role of radiotherapy was verified; a higher CR rate was observed after radiotherapy than after upfront chemotherapy, and CR to sequential CRT showed an association with improved OS, whereas CR to upfront chemotherapy did not.
In G-PCNSL-SG-1, PFS was significantly decreased by omitting WBRT [10]. An additional benefit of PFS was obtained in patients who did not achieve CR after upfront chemotherapy compared with those who did, suggesting the importance of WBRT for disease control. Similarly, in this study, the CR rate increased after WBRT and was related to better OS and PFS, because CR to sequential CRT was a significant prognosticator. Whether or not CR is achieved following upfront chemotherapy did not show an association with OS or PFS. These findings indicate that radiotherapy after upfront chemotherapy was beneficial, particularly in patients with residual disease.
The optimal dose and field of radiotherapy after chemotherapy have never been prospectively investigated. Whole brain doses of 20 to 50 Gy with or without tumor bed boost are commonly used worldwide. It is well known that volume of radiotherapy should include the whole brain due to its infiltrating nature and involved field radiotherapy alone is insufficient [16,17]. In addition, based on a historical review of the literature, some authors reported a dose dependent positive survival benefit of WBRT, although it could not be completely applicable because of somewhat old-fashioned data [13,18]. Therefore, in general, in our institution, low-dose WBRT was used, which might reduce the risk of NT and tumor bed boost was delivered in order to compensate for low whole brain dose. The possibility of tailoring the radiation dose and field understandably remains, however, it should be prospectively explored.
Use of consolidative WBRT after achievement of CR is a still controversial issue. Some authors have doubts about the necessity of WBRT in patients with CR after chemotherapy. However, the impacts on treatment outcomes and complications of consolidative WBRT have rarely been assessed in prospective PCNSL trials. With regard to omitting WBRT, many trials are still exploring and no firm conclusion regarding response-based strategies has been made. During the period in which patients in this study were treated, consolidation WBRT against microscopic residual disease was mostly preferred to salvage WBRT at the time of failure in our institution. Alternatively, radiotherapy doses were slightly reduced in CR patients, although it was not much different in the clinical setting.
Until recently, strategies for treatment of PCNSL have not been uniform universally. Five-year OS rates varying from 22% to 56% were reported [12,19]. When compared with historical data, our survival results are slightly better. In a study reported by DeAngelis et al. [1], which provided the basis for our treatment strategies and used a similar chemotherapy regimen, the 3-year OS was 52% [1], which is comparable to the 59.8% of our study. The authors used a whole brain dose of 45 Gy or 36 Gy given in 1.2 Gy fractions twice daily. We achieved a more favorable outcome using a lower whole brain dose of median 27 Gy and boost to the tumor bed. Because we excluded patients who did not receive CRT or complete radiotherapy, selection bias might have improved our survival. Nevertheless, our results are impressive and demonstrate that sequential CRT is quite effective even with a slightly lower dose of WBRT. In our previous report describing treatment administered from 1981 to 1997, doses to the whole brain ranged from 36 Gy to 50.4 Gy with a total median dose of 54 Gy to the tumor bed [20]. Median OS of 63 months in the current study was more than twice as long as that previously reported of 29 months, although chemotherapy was administered to only 16 patients (40%) in the previous study. These findings also support that our treatment strategy is fairly useful.
Some authors also investigated radiotherapy dose reduction in patients without residual disease after primary chemotherapy, considering that NT was closely associated with this factor. Shah et al. [14] reported that 2-year OS was 89% for patients treated with WBRT of 23.4 Gy after achieving CR in response to upfront chemotherapy, which was similar to patients who received WBRT of 45 Gy after obtaining non-CR in response to chemotherapy. In addition, Ferreri et al. [15] proposed that patients in CR after chemotherapy should be treated with WBRT of 30 to 36 Gy because disease control using this dose did not differ from WBRT of 40 to 44 Gy, whereas neurologic impairment was more common in patients treated after WBRT with more than 40 Gy. The authors used tumor bed boost like ours and reported a 5-year OS of 54%, which corresponded well with our results. In line with the preceding results, the results of the current study support the contention that WBRT dose can be reduced without compromising survival.
To the contrary, Bessell et al. [13], who conducted a nonrandomized prospective study using WBRT with dose reduction from 45 Gy to 30.6 Gy in patients with CR after chemotherapy, reported that reduced-dose WBRT resulted in higher relapse rate and lower OS in patients younger than 60 (3-year OS 92% vs. 60%, p=0.04). However, their CHOD/BVAM regimen could not penetrate the blood-brain barrier of the CNS, except MTX, and was thought to be less effective as being evaluated in RTOG 88-06 [21]. In addition, the MTX dose of 1.5 g/m2 was lower than doses used in recent regimens [22]. These reasons may suggest a disadvantage of low dose WBRT, although it may be not reproducible with modern chemotherapy regimens. However, potential negative effects on tumor control from a reduced-dose approach should be kept in mind.
Another strategy that has been investigated for replacement of WBRT is high-dose chemotherapy with autologous peripheral blood stem cell transplantation (APBSCT). In limited patients who are young or have good performance status, this approach can be used to escalate chemotherapy dose as well as to eliminate the need for WBRT. Preliminary studies with small numbers of patients demonstrated its feasibility, however, PFS was not satisfactory compared with that achieved with WBRT [6,23]. Heterogeneous regimens used and variable reported results also make comparison between trials difficult. Moreover, treatment toxicity remains a potential disadvantage of this approach. However, encouraging results have been reported, suggesting that high-dose chemotherapy with APBSCT may have a role in selected patients or in salvage settings because of lack of cross resistance with MTX [24]. Conduct of further studies will be needed in order to clarify the optimal indications and treatment regimens.
The NT rate in this study was lower than those reported in many other studies [4,10,25], probably due to low-dose WBRT, although direct comparison could not be made because of various examining methods and grading systems. However, our result showing an association of old age with higher risk of NT coincides well with the previous report showing that risk of NT at seven years for patients aged > 60 years was 58%, compared with 27% for those aged ≤ 60 years [3]. Despite the lack of a formal neuropsychiatric assessment, these findings supported that dose reduction of WBRT in the elderly would be helpful. Further prospective, randomized studies with appropriate neurologic function tests are needed in order to draw definitive conclusions.
There were some limitations in this study. First, this was a retrospective study, as with many other studies on PCNSL, due to its rarity. Second, the small number of patients and relatively short follow-up duration restricted statistical power. False-negative results might confound evaluation of survival and prognostic factors.
Despite these limitations, the results can be informative because we evaluated a homogeneous population of patients treated with relatively consistent methods over the period of study.
In conclusion, treatment failure and iatrogenic NT are the main obstacles in management of PCNSL patients. Findings of our study suggest that low-dose WBRT with tumor bed boost after HD-MTX based chemotherapy is an effective and reasonable solution to overcoming these problems. In addition, omitting or deferring WBRT in elderly patients could be considered because considerable incidence of NT was observed. Conduct of larger prospective studies is needed in order to confirm our results and to clarify treatment policy.
ACKNOWLEDGMENTS
This work was supported by the National R&D Program for Cancer Control by National Cancer Center Korea (grant No. 1320220) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. 2013M2A2A7043683).
References
1. DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ; Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002; 20:4643–8.

2. Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, Van't Veer M, Hansen M, Soubeyran P, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003; 21:4483–8.

3. O'Brien PC, Roos DE, Pratt G, Liew KH, Barton MB, Poulsen MG, et al. Combined-modality therapy for primary central nervous system lymphoma: long-term data from a Phase II multicenter study (Trans-Tasman Radiation Oncology Group). Int J Radiat Oncol Biol Phys. 2006; 64:408–13.
4. Correa DD, Shi W, Abrey LE, Deangelis LM, Omuro AM, Deutsch MB, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol. 2012; 14:101–8.

5. Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006; 24:4570–4.

6. Montemurro M, Kiefer T, Schuler F, Al-Ali HK, Wolf HH, Herbst R, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007; 18:665–71.
7. Hoang-Xuan K, Taillandier L, Chinot O, Soubeyran P, Bogdhan U, Hildebrand J, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003; 21:2726–31.

8. Pels H, Schmidt-Wolf IG, Glasmacher A, Schulz H, Engert A, Diehl V, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003; 21:4489–95.

9. Gerstner ER, Carson KA, Grossman SA, Batchelor TT. Longterm outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation. Neurology. 2008; 70:401–2.

10. Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010; 11:1036–47.

11. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003; 13:176–81.

12. Brada M, Hjiyiannakis D, Hines F, Traish D, Ashley S. Short intensive primary chemotherapy and radiotherapy in sporadic primary CNS lymphoma (PCL). Int J Radiat Oncol Biol Phys. 1998; 40:1157–62.

13. Bessell EM, Lopez-Guillermo A, Villa S, Verger E, Nomdedeu B, Petit J, et al. Importance of radiotherapy in the outcome of patients with primary CNS lymphoma: an analysis of the CHOD/BVAM regimen followed by two different radiotherapy treatments. J Clin Oncol. 2002; 20:231–6.

14. Shah GD, Yahalom J, Correa DD, Lai RK, Raizer JJ, Schiff D, et al. Combined immunochemotherapy with reduced wholebrain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007; 25:4730–5.

15. Ferreri AJ, Verona C, Politi LS, Chiara A, Perna L, Villa E, et al. Consolidation radiotherapy in primary central nervous system lymphomas: impact on outcome of different fields and doses in patients in complete remission after upfront chemotherapy. Int J Radiat Oncol Biol Phys. 2011; 80:169–75.

16. Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology. 2002; 59:1557–62.

17. Shibamoto Y, Hayabuchi N, Hiratsuka J, Tokumaru S, Shirato H, Sougawa M, et al. Is whole-brain irradiation necessary for primary central nervous system lymphoma? Patterns of recurrence after partial-brain irradiation. Cancer. 2003; 97:128–33.
18. Murray K, Kun L, Cox J. Primary malignant lymphoma of the central nervous system. Results of treatment of 11 cases and review of the literature. J Neurosurg. 1986; 65:600–7.
19. Schultz CJ, Bovi J. Current management of primary central nervous system lymphoma. Int J Radiat Oncol Biol Phys. 2010; 76:666–78.

20. Wu HG, Kim IH, Ha SW, Park CI, Bang YJ, Huh DS. Survival improvement with combined radio-chemotherapy in the primary central nervous system lymphomas. J Korean Med Sci. 1999; 14:565–70.

21. Schultz C, Scott C, Sherman W, Donahue B, Fields J, Murray K, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol. 1996; 14:556–64.

22. Deckert M, Engert A, Bruck W, Ferreri AJ, Finke J, Illerhaus G, et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia. 2011; 25:1797–807.

23. Colombat P, Lemevel A, Bertrand P, Delwail V, Rachieru P, Brion A, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006; 38:417–20.

Table 1.
Clinical characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Age (yr) | |
| < 60 | 37 (57.8) |
| ≥ 60 | 27 (42.2) |
| Gender | |
| Male | 38 (59.4) |
| Female | 26 (40.6) |
| Performance status (ECOG) | |
| 0-1 | 39 (60.9) |
| 2-3 | 25 (39.1) |
| Pathology | |
| Diffuse large B-cell lymphoma | 58 (90.6) |
| Small lymphocytic lymphoma | 1 (1.6) |
| Unclassified B-cell lymphoma | 1 (1.6) |
| T-cell lymphoma, NOS | 4 (6.3) |
| CSF cytology | |
| Positive | 7 (10.9) |
| Negative | 57 (89.1) |
| Operation | |
| Stereotactic biopsy | 54 (84.4) |
| Gross total resection | 7 (10.9) |
| Subtotal resection | 3 (4.7) |
| No. of lesions | |
| Single | 15 (23.4) |
| Multiple | 49 (76.6) |
| Involvement of deep structurea) | |
| Yes | 41 (64.1) |
| No | 23 (35.9) |
Table 2.
Univariate analysis for prognostic factors of overall survival (OS) and progression-free survival (PFS)
| Variable | No. of patients | 3-yr OS (%) | p-valuea) | 3-yr PFS (%) | p-valuea) |
|---|---|---|---|---|---|
| Age (yr) | |||||
| < 60 | 37 | 68.9 | 0.022 | 53.0 | 0.563 |
| ≥ 60 | 27 | 45.8 | 34.4 | ||
| Gender | |||||
| Male | 38 | 61.6 | 0.362 | 48.5 | 0.988 |
| Female | 26 | 57.2 | 45.2 | ||
| Performance status (ECOG) | |||||
| 0-1 | 39 | 69.4 | 0.012 | 48.6 | 0.975 |
| 2-3 | 25 | 43.4 | 47.9 | ||
| CSF cytology | |||||
| Positive | 7 | 42.9 | 0.097 | 25.0 | 0.472 |
| Negative | 57 | 62.1 | 50.6 | ||
| No. of lesions | |||||
| Single | 15 | 57.0 | 0.692 | 45.5 | 0.875 |
| Multiple | 49 | 61.0 | 48.6 | ||
| Involvement of deep structure | |||||
| Yes | 41 | 49.0 | 0.018 | 45.1 | 0.341 |
| No | 23 | 76.7 | 51.7 | ||
| CR to upfront chemotherapy | |||||
| Yes | 27 | 66.1 | 0.079 | 42.1 | 0.506 |
| No | 37 | 54.3 | 52.4 | ||
| CR to sequential CRT | |||||
| Yes | 55 | 63.9 | 0.019 | 50.4 | 0.009 |
| No | 9 | 33.9 | 38.1 |
Table 3.
Multivariate analysis for overall survival
Table 4.
Comparison of previous studies of PCNSL with high dose methotrexate and whole brain radiotherapy
| Study | No. of patients | WB dose to CR (Gy) | WB dose to non-CR (Gy) | Tumor bed dose (Gy) | CRR (%) | BRR (%) | Median OS (mo) | Median PFS (mo) |
|---|---|---|---|---|---|---|---|---|
| Present study | 64 | 18-36 | 19.8-36 | 45-54 | 42-86 | 40 | 63 | 34 |
| Brada et al. (1998) [12] | 31 | 30-45 | 30-45 | 40-65 | 33-67 | 44 | 23 | 5-yr, 32% |
| Bessell et al. (2002) [13] | 57 | 30.6 | 45 | 30.6-55 | 62-77 | NR | 5-yr, 36% | NR |
| DeAngelis et al. (2002) [1] | 98 | 36-45 | 45 | 45 | 58-NR | 26 | 36.9 | 24 |
| Poortmans et al. (2003) [2] | 52 | 30-40 | 39-40 | 39-50 | 33-69 | 27 | 46 | NR |
| O’Brien et al.(2006) [3] | 46 | 45-50.4 | 45-50.4 | 50.4 | NR-82 | NR | 36 | 20 |
| Shah et al. (2007) [14] | 30 | 23.4 | 45 | 23.4-45 | 44-77 | NR | 2-yr, 67% | 40 |
| Ferreri et al. (2011) [15] | 33 | 30-45 | - | 36-54 | NR | 30 | 5-yr, 54% | 5-yr, 51% |
PCNSL, primary central nervous system lymphoma; WB, whole brain; CR, complete response; CRR, complete response rate (values indicate response rate after chemotherapy-response rate after chemoradiotherapy); BRR, brain relapse rate; OS, overall survival; PFS, progression free survival; NR, not reported.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download