Introduction
Worldwide, liver cancer is the fifth most common cancer in men (16.0 per 100,000) and the seventh in women (6.0 per 100,000), with almost 85% of cases occurring in Asia and Africa [
1]. Due to its high fatality, liver cancer is the third most common cause of cancer death worldwide [
1]. Although the incidence of liver cancer in Korea has declined over the last decade, it is still the fourth most common cancer in Korean men (37.7 per 100,000) and the seventh most common cancer in Korean women (10.4 per 100,000) [
2]. In addition, liver cancer is the second most common cause of cancer death in Korea [
2].
In an effort to reduce liver cancer-related mortality, surveillance or screening is widely practiced and generally recommended for certain high-risk groups. Liver cancer occurs in populations with a well-defined set of risk factors and has a protracted preclinical phase, meaning that timely identification of disease can lead to appropriate treatment at a more curable stage; therefore, it is a suitable target for a surveillance program. The carcinogenic effect of chronic infection with hepatitis B and C viruses (HBV, HCV) in liver cancer development has been well demonstrated by epidemiological and experimental evidence. However, evidence on efficacy of liver cancer screening (or surveillance) programs has not yet been established. A number of screening programs have been reported since the 1970s, and tumors detected through screening were found to be smaller, resulting in increased survival [
3-
6]. However, in all of these studies, the duration of follow-up was limited, and lead-time bias remained. A randomized controlled trial from conducted in Shanghai using abdominal ultrasound and alpha-fetoprotein (AFP) every six months in 18,816 patients aged 35-59 years with chronic hepatitis B and other risk factors for hepatocellular carcinomas (HCC) showed a reduction in mortality by 37% [
7]. While these results are promising, the confidence interval (CI) was near 1.0, intention-to-treat analysis was not used, assessment of outcome was not blinded, and generalizability to other populations is uncertain [
8]. Therefore, while screening with AFP+ultrasonography (US) appears to detect significantly more HCC compared with no screening, and, despite the current recommendation to screen subjects at moderate and high risk for HCC every six months, we do not yet know with certainty whether screening can reduce all-cause mortality or HCC mortality, which modality of screening should be used (no screening, AFP, US, or AFP+US), or how frequently screening should be offered. It is possible that HCC screening may be effective, but also that harm caused by screening may outweigh any benefit [
9]. The National Cancer Institute reported that liver cancer screening would not result in mortality reduction from HCC [
8].
Despite the lack of concrete evidence, screening for liver cancer is widely practiced and recommended for certain at-risk groups [
10,
11]. In Korea, a nationwide liver cancer screening program was introduced in 2003 as part of the National Cancer Screening Program (NCSP). It was based on the HCC Surveillance Recommendations developed by the National Cancer Center and the Korean Association for the Study of the Liver in 2001 [
12]. The NCSP for liver cancer in Korea provides US and AFP testing at six-month intervals to men and women aged 40 years or older with chronic HBV or HCV infection, liver cirrhosis, or chronic liver disease of any cause (i.e., high-risk population). This screening strategy is the most widely accepted and the most used in clinical practice. However, the optimal screening interval and proper age range for screening are still being debated.
A cancer screening program should be cost-effective and revised according to documented epidemiological changes of the cancer in question, and development of diagnostic technology, especially when conducted at a national level. However, few studies have investigated the cost-effectiveness of liver cancer screening by consideration of various screening intervals and age ranges. Therefore, this study was conducted in order to determine the most cost-effective strategy for men and women, in terms of interval and age range, for liver cancer screening by combined US and AFP testing in the high-risk population in Korea.
Go to :

Materials and Methods
1. Model
To determine the most cost-effective interval and age range for liver cancer screening, the effectiveness and the cost of screening strategies were based on a model proposed by Lee and Zelen [
13]. The optimal screening strategy was determined based on the incremental cost-effectiveness ratio (ICER), which is defined as the ratio of changes in cost and effectiveness of one screening strategy to an alternative strategy.
The effectiveness of a screening strategy was measured as the number of early-detected liver cancers found per 100,000 high-risk individuals screened. This number was derived from the estimated probability of detecting preclinical liver cancer, a state in which the disease has no symptoms but can be diagnosed. The model by Lee and Zelen [
13] assumes that the natural history of a chronic disease or cancer is progressive in the manner of
S0→
Sp→
Sc, where S0 represents
Sp the cancer-free state,
Sp the preclinical state, and
Sc the clinical state. The sensitivity of the screening method, the distribution of the mean sojourn time (MST) in the preclinical state, and age-specific incidence rates were required for estimation of the probability of preclinical detection. MST is the duration of the pre-clinical detectable phase of the cancer, and, in the current study, it was defined as the mean time necessary for a liver tumor to change from a screening detectable size to a clinically detectable size.
We assumed that both the MST and the sensitivity of the screening method (combined US and AFP testing) were constant, as assumed by the Lee and Zelen model [
13]. In the model, the time variable
t refers to age,
r refers to screening round, and i refers to screening interval. Assuming that screenings are performed at age
t1≤
t2 <…<
tn in a given period, the probability of detecting preclinical cancer at age t
r is as follows:
, where w(x) denotes the probability of progression from S0 to Sp, which can be calculated by age-specific liver cancer incidence and an assumed distribution of MST in Sp: Q(t) is the survival distribution of the MST in Sp at age t with tr-1≤t<tr, and β is the sensitivity of the modality based on the combination of US and AFP testing.
The costs were measured as the direct cost incurred when one individual underwent screening, which is reasonable for low-incidence disease [
13]. Costs of screening and confirmative tests for false-positive outcomes were included. As combined US and AFP testing is used in liver cancer screening in Korea, the costs were calculated as follows:
, where n denotes the total number of screenings, Ks and Sp represent the screening cost and specificity, respectively, for the combination of US and AFP testing, and Kd the cost of confirmative testing. Costs of other adverse effects from liver cancer screening, such as discomfort from examination or recall of patients for additional imaging, were not considered in the model. The time horizon for the study was 30 to 80 years of age. We adopted the perspective of the national healthcare system.
For the current study we generated 36 possible combinations of screening intervals and age ranges for both men and women, setting screening intervals of six months, one year, or two years; an initial screening age of 30, 40, or 50 years; and a ceiling screening age of 65, 70, 75, or 80 years (
Table 1). ICER was calculated for determination of the most cost-effective screening strategy for liver cancer among all those considered. Based on the calculated ICER, screening strategies were classified as non-dominated, dominated, or extended dominated. Non-dominated strategies, in which the effectiveness was higher and the costs were lower than others, were considered the most cost-effective. A dominated strategy was defined as generating worse effects and higher costs than an alternative strategy. Extended dominance occurred when a strategy was less effective and had a higher ICER than an alternative strategy.
Table 1.
Strategies generated for liver cancer screening by combined ultrasonography and alpha-fetoprotein testing for the cost-effectiveness analysis
|
Interval (yr) |
Initial age (yr) |
Ceiling age (yr) |
Strategy |
No. of screenings |
|
0.5 |
30 |
65 |
S_0.5_3065 |
72 |
|
70 |
S_0.5_3070 |
82 |
|
75 |
S_0.5_3075 |
92 |
|
80 |
S_0.5_3080 |
102 |
|
40 |
65 |
S_0.5_4065 |
52 |
|
70 |
S_0.5_4070 |
62 |
|
75 |
S_0.5_4075 |
72 |
|
80 |
S_0.5_4080 |
82 |
|
50 |
65 |
S_0.5_5065 |
32 |
|
70 |
S_0.5_5070 |
42 |
|
75 |
S_0.5_5075 |
52 |
|
80 |
S_0.5_5080 |
62 |
|
1 |
30 |
65 |
S_1_3065 |
36 |
|
70 |
S_1_3070 |
41 |
|
75 |
S_1_3075 |
46 |
|
80 |
S_1_3080 |
51 |
|
40 |
65 |
S_1_4065 |
26 |
|
70 |
S_1_4070 |
31 |
|
75 |
S_1_4075 |
36 |
|
80 |
S_1_4080 |
41 |
|
50 |
65 |
S_1_5065 |
16 |
|
70 |
S_1_5070 |
21 |
|
75 |
S_1_5075 |
26 |
|
80 |
S_1_5080 |
31 |
|
2 |
30 |
65 |
S_2_3065 |
18 |
|
70 |
S_2_3070 |
21 |
|
75 |
S_2_3075 |
23 |
|
80 |
S_2_3080 |
26 |
|
40 |
65 |
S_2_4065 |
13 |
|
70 |
S_2_4070 |
16 |
|
75 |
S_2_4075 |
18 |
|
80 |
S_2_4080 |
21 |
|
50 |
65 |
S_2_5065 |
8 |
|
70 |
S_2_5070 |
11 |
|
75 |
S_2_5075 |
13 |
|
80 |
S_2_5080 |
16 |

2. Data and model assumptions
For estimation of age-specific liver cancer incidence rates for the high-risk population, the high-risk population in the NCSP database in 2008 was linked to the 2008 Korean National Cancer Incidence Database of the Korean Central Cancer Registry (KCCR). In the NCSP, the National Health Insurance (NHI) Corporation identified the high-risk group for liver cancer screening as individuals who had been tested or received medical care for HBV or HCV infection (ICD 10 code: B18, B18.0, B18.1, B18.2, Z22.5), chronic liver disease (ICD 10 code: B19, K73, K73.1, K73.2. K73.8, K73.9), or liver cirrhosis (ICD 10 code: K74, K74.1, K74.2, K74.6, K76, K70.2, K70.3, K70.9) within the past two years, using the computerized medical claims database. Incidence rates were calculated for each age group and ranged from 30 to 34 years of age to more than 85 years of age. The incidence of liver cancer in the high risk group showed a gradual increase with age until the age 80-84 years and then subsequently decreased. We assumed that individuals in the high-risk population aged 30 or over undergo liver cancer screening and are followedup until the ceiling screening age. Therefore, in the model, high-risk individuals remained in the screening cohort and underwent screening based on the set screening interval until reaching the ceiling screening age (65, 70, 75, or 80 years).
Baseline assumptions regarding MST, sensitivity and specificity, and cost of screening and confirmative tests are shown in
Table 2. A previous study conducted in Taiwan reported an MST of 1.57 years (95% CI, 0.94 to 4.68 years) in cirrhotic patients and 2.66 years (95% CI, 1.68 to 6.37 years) in non-cirrhotic patients [
3]. Another study conducted in Asia reported an MST of 3.2 years, regardless of the severity of cirrhosis [
14]. In the current study, based on the abovementioned study conducted in Taiwan, we assumed an MST of 1.57 years for the high-risk population (i.e., chronic HBV or HCV infection, liver cirrhosis, or chronic liver disease of any cause). Sensitivity and specificity of US are known to range from 65% to 84% and from 91% to 97%, respectively [
6,
15]. The reported sensitivity of AFP has ranged from 39% to 69% with the standard of 20 ng/mL, and specificity from 90% to 95% [
15,
16]. Sensitivity increased up to 92% when US and AFP were combined [
15]. However, due to differences in target populations and liver cancer screening programs, direct use of these results may be difficult.
Table 2.
Baseline assumptions and ranges tested in the sensitivity analysis
|
Parameters |
Baseline model |
Sensitivity analysis |
Reference |
|
Mean sojourn time (yr) |
1.57 |
0.94-4.68 |
7 |
|
|
(0.94, 2, 2.66, 4.68) |
|
|
Screening testa)
|
|
|
|
|
Sensitivity (%) |
50 |
60, 70 |
16 |
|
Specificity (%) |
95 |
90 |
16 |
|
Unit cost (US$) |
|
|
|
|
Screening testa)
|
52.28b)
|
|
17 |
|
Confirmative test |
311.09c)
|
|
17 |

For example, most of the aforementioned studies were hospital-based, and, in general, the sensitivity and specificity of these tests are lower in a community-based setting with an asymptomatic population. A previous study reported sensitivity and specificity of combined US and AFP testing in the NCSP ranging from 42% to 54% and from 94% to 96%, respectively [
17]. Thus, this study assumed a sensitivity and specificity of combined US and AFP testing of 50% and 95%, respectively. The unit costs of US (US$41.29), AFP (US$ 10.99), and the confirmative test (magnetic resonance imaging, US$311.09) were obtained from the 2009 medical insurance costs published by the Health Insurance Review and Assessment Service [
18], and combined for estimation of total costs. To assess the robustness of the proposed screening strategies, one-way sensitivity analyses were performed by changing the MST in the preclinical state, and the sensitivity and specificity of combined US and AFP testing. Alternative MSTs were 0.94, 2.66, 4.68, and 6.37 years based on the previous study [
3], alternative sensitivities were 60% and 70%, and specificity was 90%. All statistical analyses were performed using MATLAB 6.1 (Mathworks Inc., Natick, MA).
Go to :

Results
Tables 3 and
4 show the 36 strategies for liver cancer screening by US and AFP in men and women in the high-risk population, as well as the number of cases found per 100,000 high-risk individuals screened, cost per 100,000 screenings, cost per preclinical case detected, incremental cases found, incremental cost, and ICER. They are listed in ascending order of cost per 100,000 screenings. The most expensive strategy, with a six-month interval and an age range of 30 to 80 years for men and women, found 5,440 and 2,212 preclinical cases per 100,000 high-risk individuals screened, respectively. The least expensive screening plan, consisting of a screening interval of two years for men and women with an age range of 50 to 65 years, detected 915 and 338 preclinical cases per 100,000 high-risk individuals screened, respectively.
Table 3.
Cost effectiveness of strategies for liver cancer screening by combined ultrasonography and alpha-fetoprotein testing in Korean men
|
Strategy |
Cases founda) per 100,000 screened |
Cost per 100,000 screenings (US$) |
Cost/Case detected (US$) |
Incremental cases foundb)
|
Incremental costc)
|
ICERd)
|
Category of dominance |
|
S2_5065 |
914.7 |
43,379,435 |
47,425 |
914.7 |
43,379,435 |
47,425 |
E. dominated |
|
S2_5070 |
1,319.9 |
59,063,435 |
44,748 |
1,319.9 |
59,063,435 |
44,748 |
E. dominated |
|
S2_4065 |
1,264.3 |
69,519,435 |
54,987 |
- |
- |
- |
Dominated |
|
S2_5075 |
1,633.2 |
69,519,435 |
42,566 |
1,633.2 |
69,519,435 |
42,566 |
E. dominated |
|
S2_4070 |
1,669.5 |
85,203,435 |
51,035 |
1,669.5 |
85,203,435 |
51,035 |
E. dominated |
|
S2_5080 |
2,088.2 |
85,203,435 |
40,802 |
2,088.2 |
85,203,435 |
40,802 |
- |
|
S1_5065 |
1,511.9 |
85,203,435 |
56,355 |
- |
- |
- |
Dominated |
|
S2_3065 |
1,464.7 |
95,659,435 |
65,310 |
- |
- |
|
Dominated |
|
S2_4075 |
1,982.8 |
95,659,435 |
48,245 |
- |
- |
- |
Dominated |
|
S2_3070 |
1,869.9 |
111,343,435 |
59,545 |
- |
- |
- |
Dominated |
|
S2_4080 |
2,437.7 |
111,343,435 |
45,676 |
349.5 |
26,140,000 |
74,793 |
E. dominated |
|
S1_5070 |
2,015.8 |
111,343,435 |
55,235 |
- |
- |
- |
Dominated |
|
S2_3075 |
2,183.2 |
121,799,435 |
55,789 |
- |
- |
- |
Dominated |
|
S2_3080 |
2,638.2 |
137,483,435 |
52,113 |
550.0 |
52,280,000 |
95,055 |
E. dominated |
|
S1_4065 |
2,038.3 |
137,483,435 |
67,450 |
- |
- |
- |
Dominated |
|
S1_5075 |
2,615.6 |
137,483,435 |
52,563 |
- |
- |
- |
Dominated |
|
S1_4070 |
2,542.3 |
163,623,435 |
64,360 |
- |
- |
- |
Dominated |
|
S1_5080 |
3,192.4 |
163,623,435 |
51,254 |
1,104.2 |
78,420,000 |
71,020 |
- |
|
S0.5_5065 |
2,035.8 |
168,851,435 |
82,941 |
- |
- |
- |
Dominated |
|
S1_3065 |
2,345.7 |
189,763,435 |
80,898 |
- |
- |
- |
Dominated |
|
S1_4075 |
3,142.0 |
189,763,435 |
60,396 |
- |
- |
- |
Dominated |
|
S1_3070 |
2,849.6 |
215,903,435 |
75,766 |
- |
- |
- |
Dominated |
|
S1_4080 |
3,718.9 |
215,903,435 |
58,056 |
526.5 |
52,280,000 |
99,297 |
- |
|
S0.5_5070 |
2,707.7 |
221,131,435 |
81,668 |
- |
- |
- |
Dominated |
|
S1_3075 |
3,449.4 |
242,043,435 |
70,170 |
- |
- |
- |
Dominated |
|
S1_3080 |
4,026.2 |
268,183,435 |
66,610 |
307.3 |
52,280,000 |
170,127 |
E. dominated |
|
S0.5_4065 |
2,740.4 |
273,411,435 |
99,771 |
- |
- |
- |
Dominated |
|
S0.5_5075 |
3,512.6 |
273,411,435 |
77,837 |
- |
- |
- |
Dominated |
|
S0.5_4070 |
3,412.2 |
325,691,435 |
95,449 |
- |
- |
- |
Dominated |
|
S0.5_5080 |
4,284.0 |
325,691,435 |
76,025 |
565.1 |
109,788,000 |
194,281 |
E. dominated |
|
S0.5_3065 |
3,151.7 |
377,971,435 |
119,926 |
- |
- |
- |
Dominated |
|
S0.5_4075 |
4,217.2 |
377,971,435 |
89,626 |
- |
- |
- |
Dominated |
|
S0.5_3070 |
3,823.5 |
430,251,435 |
112,528 |
- |
- |
|
Dominated |
|
S0.5_4080 |
4,988.5 |
430,251,435 |
86,249 |
1,269.6 |
214,348,000 |
168,831 |
- |
|
S0.5_3075 |
4,628.5 |
482,531,435 |
104,252 |
- |
- |
- |
Dominated |
|
S0.5_3080 |
5,399.8 |
534,811,435 |
99,043 |
411.3 |
104,560,000 |
254,218 |
- |

Table 4.
Cost effectiveness strategies for liver cancer screening by combined ultrasonography and alpha-fetoprotein testing in Korean women
|
Strategy |
Cases founda) per 100,000 screened |
Cost per 100,000 screenings (US$) |
Cost/Case detected (US$) |
Incremental cases foundb)
|
Incremental costc)
|
ICERd)
|
Category of dominance |
|
S2_5065 |
337.5 |
43,379,435 |
128,532 |
337.5 |
43,379,435 |
128,532 |
E. dominated |
|
S2_5070 |
539.6 |
59,063,435 |
109,458 |
539.6 |
59,063,435 |
109,458 |
E. dominated |
|
S2_4065 |
407.3 |
69,519,435 |
170,684 |
- |
- |
- |
Dominated |
|
S2_5075 |
694.6 |
69,519,435 |
100,086 |
694.6 |
69,519,435 |
100,086 |
E. dominated |
|
S2_4070 |
565.8 |
85,203,435 |
150,589 |
- |
- |
- |
Dominated |
|
S2_5080 |
609.4 |
85,203,435 |
139,815 |
- |
- |
- |
Dominated |
|
S1_5065 |
978.8 |
85,203,435 |
87,049 |
978.8 |
85,203,435 |
87,049 |
- |
|
S2_3065 |
441.6 |
95,659,435 |
216,620 |
- |
- |
- |
Dominated |
|
S2_4075 |
764.5 |
95,659,435 |
125,127 |
- |
- |
- |
Dominated |
|
S2_3070 |
643.7 |
111,343,435 |
172,974 |
- |
- |
- |
Dominated |
|
S2_4080 |
821.7 |
111,343,435 |
135,504 |
- |
- |
- |
Dominated |
|
S1_5070 |
1,048.7 |
111,343,435 |
106,173 |
69.9 |
26,140,000 |
373,963 |
E. dominated |
|
S2_3075 |
798.7 |
121,799,435 |
152,497 |
- |
- |
- |
Dominated |
|
S2_3080 |
672.8 |
137,483,435 |
204,345 |
- |
- |
- |
Dominated |
|
S1_4065 |
1,082.9 |
137,483,435 |
126,959 |
104.1 |
52,280,000 |
502,209 |
E. dominated |
|
S1_5075 |
1,118.5 |
137,483,435 |
122,918 |
35.6 |
52,280,000 |
374,230 |
E. dominated |
|
S1_4070 |
928.7 |
163,623,435 |
176,185 |
- |
- |
- |
Dominated |
|
S1_5080 |
1,488.1 |
163,623,435 |
109,955 |
509.3 |
78,420,000 |
153,976 |
- |
|
S0.5_5065 |
762.6 |
168,851,435 |
221,415 |
- |
- |
|
Dominated |
|
S1_3065 |
724.9 |
189,763,435 |
261,779 |
- |
- |
- |
Dominated |
|
S1_4075 |
1,225.5 |
189,763,435 |
154,846 |
- |
- |
- |
Dominated |
|
S1_3070 |
980.8 |
215,903,435 |
220,130 |
- |
- |
- |
Dominated |
|
S1_4080 |
1,595.1 |
215,903,435 |
135,354 |
107.0 |
52,280,000 |
488,598 |
E. dominated |
|
S0.5_5070 |
1,104.7 |
221,131,435 |
200,173 |
- |
- |
- |
Dominated |
|
S1_3075 |
1,277.7 |
242,043,435 |
189,437 |
- |
- |
- |
Dominated |
|
S1_3080 |
1,647.3 |
268,183,435 |
162,802 |
159.2 |
104,560,000 |
656,784 |
E. dominated |
|
S0.5_4065 |
905.8 |
273,411,435 |
301,845 |
- |
- |
- |
Dominated |
|
S0.5_5075 |
1,502.9 |
273,411,435 |
181,923 |
- |
- |
- |
Dominated |
|
S0.5_4070 |
1,248.0 |
325,691,435 |
260,971 |
- |
- |
- |
Dominated |
|
S0.5_5080 |
1,999.0 |
325,691,435 |
162,927 |
510.9 |
162,068,000 |
317,221 |
- |
|
S0.5_3065 |
975.6 |
377,971,435 |
387,425 |
- |
- |
- |
Dominated |
|
S0.5_4075 |
1,646.1 |
377,971,435 |
229,616 |
- |
- |
- |
Dominated |
|
S0.5_3070 |
1,317.7 |
430,251,435 |
326,517 |
- |
- |
- |
Dominated |
|
S0.5_4080 |
2,142.2 |
430,251,435 |
200,846 |
143.2 |
104,560,000 |
730,168 |
- |
|
S0.5_3075 |
1,715.9 |
482,531,435 |
281,212 |
- |
- |
- |
Dominated |
|
S0.5_3080 |
2,212.0 |
534,811,435 |
241,777 |
69.8 |
104,560,000 |
1,497,994 |
- |

Among the 36 alternative strategies for men, five (S_2_5080, S_1_5080, S_1_4080, S_0.5_4080, and S_0.5_3080) were identified as non-dominated strategies, and others were eliminated by either simple or extended dominance. The least expensive strategy was compared to a plan in which no screening was performed. The two-year or one-year interval non-dominated screening plans for the age range of 50 to 80 years had an ICER of US$40,802, US$71,020 per case detected, respectively. However, two-year non-dominated screening plans showed fewer cases per 100,000 screened, which was less than 40% of cases from the most expensive strategy (S_0.5_3080). Compared to a two-year interval plan for the 50-80 year age group, extending one-year screening for the age range of 50 to 80 (S_1_5080) was the next non-dominated strategy with an ICER of US$71,020 per one case found. The non-dominated screening plans above S_1_4080, such as S_0.5_4080 and S_0.5_3080 were not relatively cost-effective because the costs of these strategies were five times higher than the least expensive strategy (
Table 3).
For women, identified non-dominated strategies were as follows: S_1_5065, S_1_5080, S_0.5_5080, S_0.5_4080, and S_0.5_3080 (
Table 4). However, the ICERs of non-dominated strategies for women were at least two times higher than those for men. Compared to a one-year interval for the age range of 50 to 65 years (S_1_5065), extending one-year screening to age group 50-80 resulted in an increase in ICER from US$87,049 to US$153,976 (
Table 4).
Fig. 1 illustrates our expansion path results consisting of the most cost-effective screening strategies. The expansion path graph, which plots the expected number of detected cases against the costs of each screening strategy, was illustrated based on the ICER shown in
Tables 3 and
4. The graph representing the ICER shows a slow increase up to the S_1_4080 strategy for men and S_1_5080 strategy for women, but a steep increase at the S_0.5_4080 strategy for men and S_0.5_5080 strategy for women. In other words, the graph visually demonstrates that strategies above S_1_4080 for men and S1_5080 for women required large additional costs in order to achieve increasing effectiveness. Considering the ICER and the relative detection probability in the preclinical stage, the S_1_5080 and S_1_4080 strategies were chosen as the most cost-effective strategies for Korean men. For women, the ICER showed a steeper increase than for men, more than two times higher.
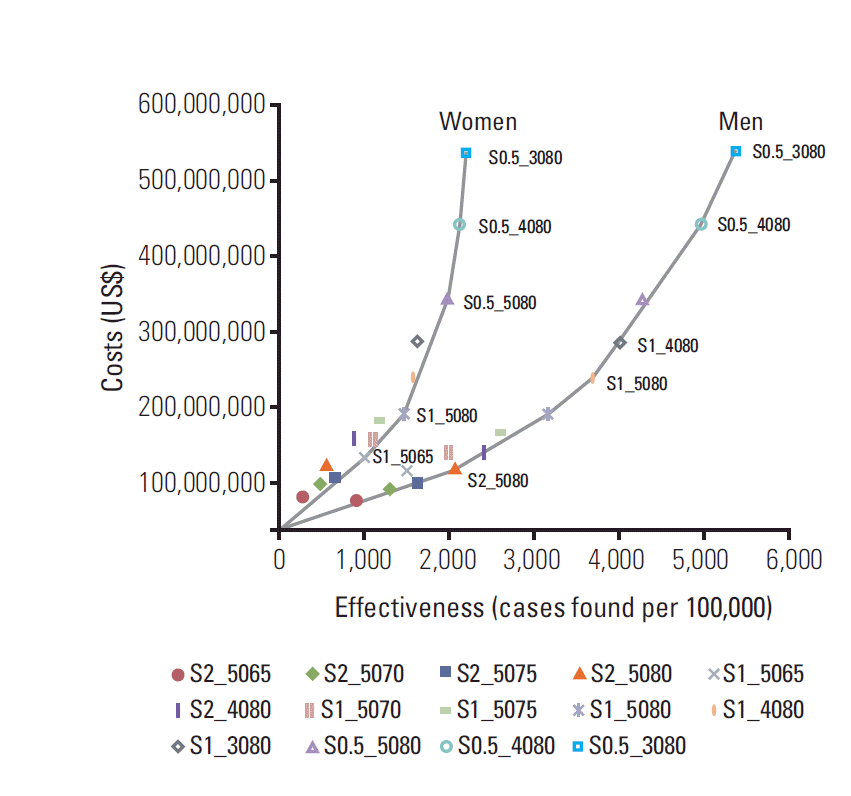 | Fig. 1.Expansion path graph of the most cost-effective strategies for liver cancer screening by combined ultrasonography and alpha-fetoprotein in Korean men and women. 
|
Sensitivity analyses of the identified cost-effective strategies for men and women were performed based on the different parameter settings (
Table 5). The non-dominated strategies selected from each model for sensitivity showed consistency with those from the baseline model (data not shown). According to the various values of sensitivity parameters, relative ratio of cases to base-line model ranged from 100% to 124% of the baseline model for men and women, except the mean sojourn time. Cases found per 100,000 screened for the different values of mean sojourn time varied from 73% to 191% of the baseline model.
Table 5.
Sensitivity analysis of strategies for liver cancer screening by combined ultrasonography and alpha-fetoprotein testing in Korean men and women
|
S1_5080
|
S0.5_4080
|
|
Cases founda) per 100,000 screened (%)b)
|
Cost per 100,000 screenings (%)b)
|
Cases founda) per 100,000 screened (%)b)
|
Cost per 100,000 screenings (%)b)
|
|
Men |
|
|
|
|
|
Baseline model |
3,192.4 (100) |
163,623,435 (100) |
4,988.5 (100) |
430,251,435 (100) |
|
Mean sojourn time (yr) |
|
|
|
|
|
0.94 |
2,353.9 (74) |
163,623,435 (100) |
4,021.4 (81) |
430,251,435 (100) |
|
2.66 |
4,061.2 (127) |
163,623,435 (100) |
5,901.2 (118) |
430,251,435 (100) |
|
4.68 |
4,921.3 (154) |
163,623,435 (100) |
6,808.1 (137) |
430,251,435 (100) |
|
6.37 |
5,357.9 (168) |
163,623,435 (100) |
7,311.3 (147) |
430,251,435 (100) |
|
Sensitivity of screening testc)
|
|
|
|
|
|
60% |
3,585.7 (112) |
163,623,435 (100) |
5,385.6 (108) |
430,251,435 (100) |
|
70% |
3,931.5 (123) |
163,623,435 (100) |
5,710.2 (115) |
430,251,435 (100) |
|
Specificity of screening testc)
|
|
|
|
|
|
90% |
3,192.4 (100) |
165,178,870 (101) |
4,988.5 (100) |
431,806,870 (100) |
|
Women |
|
|
|
|
|
Baseline model |
1,488.1 (100) |
163,623,435 (100) |
2,142.2 (100) |
430,251,435 (100) |
|
Mean sojourn time (yr) |
|
|
|
|
|
0.94 |
1,078.9 (73) |
163,623,435 (100) |
1,706.1 (80) |
430,251,435 (100) |
|
2.66 |
1,953.4 (131) |
163,623,435 (100) |
2,588.7 (121) |
430,251,435 (100) |
|
4.68 |
2,502.0 (168) |
163,623,435 (100) |
3,094.7 (145) |
430,251,435 (100) |
|
6.37 |
2,839.6 (191) |
163,623,435 (100) |
3,409.5 (159) |
430,251,435 (100) |
|
Sensitivity of screening testc)
|
|
|
|
|
|
60% |
1,673.6 (113) |
163,623,435 (100) |
2,315.8 (108) |
430,251,435 (100) |
|
70% |
1,837.1 (124) |
163,623,435 |
(100) 2,457.9 (115) |
430,251,435 (100) |
|
Specificity of screening testc)
|
|
|
|
|
|
90% |
1,488.1 (100) |
165,178,870 (101) |
2,142.2 (100) |
431,806,870 (100) |

Go to :

Discussion
There is a general consensus among researchers and clinicians that liver cancer screening in high-risk groups has the potential to significantly reduce mortality [
3,
7]. However, due to a lack of information on its cost-effectiveness, there are limitations to introduction of liver cancer screening as a nationwide program. Optimal screening interval and the initial and ceiling screening age are the two major issues in liver cancer screening, as these factors are directly related to the detection rate of preclinical liver cancer, as well as the total cost of a program.
The optimal screening interval should be determined by tumor growth rate. An interval that is too long would allow tumors to grow to an extent that would preclude curative treatment. The mean tumor volume doubling time was estimated as 117-127 days (95% confidence interval, 80 to 203 days) [
14,
19]. Based on these tumor doubling times, a screening interval of 4-12 months has been suggested [
14]. However, a retrospective study conducted on cirrhotic patients reported no differencein survival in patients screened at six- or 12-month intervals [
20]. In addition, one study used a mathematical analysis for the tumor growth rate among HBV carriers, and showed that screening at 10-month or one-year intervals was the most cost-effective [
21]. However, in studies of high-risk populations for liver cancer, screening at six-month intervals resulted in improved survival compared to one-year intervals [
20,
22]. Although most experts use a six-month interval, there are no clear data to suggest that it is the most-cost effective screening interval. In the current study, we explored three screening intervals: six months, one year, or two years, and the results showed that a one-year interval was the most cost-effective for both men and women.
Cost-effectiveness can be improved if screening is limited to well-defined groups of patients. In the case of liver cancer, chronic HBV and HCV infection are recognized as major risk factors for liver cancer. Cirrhosis is also a risk factor for liver cancer, irrespective of etiology, as is increasing age and male gender. However, only a limited number of studies have addressed the question of whether screening for liver cancer should or could be restricted to individuals within a certain age range. The initial screening age should be determined by the relationship between age and the incidence rate of liver cancer. In Korea, due to the high prevalence of HBV infection and related liver problems, the government introduced an HBV vaccination program in 1983; the vaccine was offered to government employees, soldiers, and students on a voluntary basis. In 1995, a national HBV vaccination program for infants and children was launched, followed by a 2002 national vaccination program directed at HBV-infected mothers for prevention of vertical transmission to their newborns. Thereafter, hepatitis B surface antigen seropositivity among members of the Korean population under 20 years of age showed a dramatic decrease to 2% [
23]. Currently, most members of the Korean population under 20 years of age were born after introduction of the HBV vaccination program. Compared to this population, Koreans over 30 years of age have a higher prevalence of HBV infection. In the current study, we estimated the age-specific liver cancer incidence rates for the high-risk population between ages 30 and 80 years. Based on these incidence data, our results showed that the most cost-effective age range for liver cancer screening was from 50 to 80 for men and women.
The purpose of our study was to determine the most costeffective screening interval and age range based on the epidemiological characteristics of liver cancer in Korean men and women. In Korea, the entire population is enrolled in the mandatory NHI program, and liver cancer screening sponsored by the NHI is free of charge for most beneficiaries. Therefore, we performed the analysis from the perspective of the national healthcare system as we were primarily concerned with determining direct payments from the government. In this study, we did not measure indirect costs or lost productivity associated with liver cancer.
The effectiveness of a cancer screening program is assessed by decreases in cancer mortality rates; therefore, life-year gained (LYG), quality-adjusted life-years (QALY), or disability-adjusted life-years (DALY) are often used as the final endpoint for evaluation of screening strategies [
24]. Although there is no consensus on a cost-effectiveness threshold below which an intervention would be considered unequivocally cost effective, a value of US$50,000/QALY is often cited as a benchmark [
24]. On the whole, liver cancer screening in Korea might not be as cost-effective based on the threshold of US$50,000. However, we should consider that the cost effectiveness threshold is often referred to as society’s willingness topay for an additional unit of health gain (i.e., cancer detection). Thus, in the current study, interventions were classified based on the level of cost-effectiveness by convention, as described in the literature [
25]: cost saving (an intervention generates a better health outcome and costs less than the comparison intervention) or cost neutral (ICER=0); very cost-effective (0<ICER≤US$25,000); cost-effective (US$25,000<ICER≤US$50,000); marginally cost-effective (US$50,000<ICER≤US$100,000); or not cost-effective (US$100,000). In the current study, considering the high burden of liver cancer in Korea, we set a threshold of ICER≤US$100,000 (marginally cost-effective). In this regard, the relatively cost-effective strategies were identified in the analysis: 1) two-year interval for men in the age range 50 to 80 years (ICER, US$40,802); 2) one-year interval for men in the age range 50 to 80 years (ICER, US$71,020); 3) one-year screening interval for men in the age range 40 to 80 years (ICER, US $99,297); 4) one-year interval for women in the age range 50 to 65 years (ICER, US$87,049).
Our study has several limitations. One was the hypothesis that the rate of participation in liver cancer screening would be the same for all age ranges. In reality, different screening intervals and age ranges could affect the participation rate and in turn the cost-effectiveness analysis, depending on the strategies chosen. Conduct of afuture study will be needed in order to reflect the participation rate in the model.
In addition, we utilized the Korean National Cancer Incidence Database and NCSP database for estimation of the age-specific incidence rates of liver cancer in the high risk group. Because the voluntarily participating hospitals in KCCR could not cover all new cases in the entire country, the incidence rates could be underestimated. In addition, the high risk group for liver cancer identified from the NCSP database could not cover all high risk populations, because in the NCSP, individuals who had been tested or received medical care for HBV or HCV infection, chronic liver disease, or liver cirrhosis within the past two years were selected as the high risk group for liver cancer. However, underestimation of incidence itself does not influence the estimate of the number of examinations because the age-specific incidence rates are not unevenly underestimated over all ages.
In addition, we do not have data on the MST for liver cancer in Korea. As a result, the analysis had to be performed using reference data from Taiwan [
3], which were assumed to be similar to Korean data. In the analysis, we did not distinguish between screening strategies for cirrhotic and non-cirrhotic patients, as we could not estimate liver cancer incidence rates for these groups separately. In addition, in the screening program at the national level, adoption of different screening strategies according to an individual’s underlying condition (i.e., cirrhotic or non-cirrhotic) is very difficult. The NCSP for liver cancer in Korea provides the same screening services to both cirrhotic and non-cirrhotic patients: consequently, we simply adopted a conservative MST value of 1.57 years in the baseline model for determination of the most cost-effective strategy for liver cancer screening, in terms of interval and age range for the defined high-risk population in Korea. We also performed sensitivity analyses by changing the MST in the preclinical state.
Finally, to identify the most cost-effective screening interval and target age range, we considered cases detected at the preclinical state as potential surrogate outcomes in screening strategies instead of LYG or QALY. The cost-effectiveness model using mortality reduction as an indicator of effectiveness (LYG, QALY, and DALY) mostly demands various parameters, such as progressive transition probabilities from state to state, fatality rate for each state, and medical costs related to treatment methods, such as transplantation, transarterial chemoembolization, radio frequency ablation, and so on. However, in order to obtain adequate statistical estimates, conduct of long follow-up clinical trials with a sufficiently large number of subjects would be required. As we were not able to obtain a sufficient amount of the data mentioned above on liver cancer in Korea, using the number of preclinical cases detected might be the best alternative for measuring effectiveness in this study. In addition, evidence for decreased liver cancer mortality following early detection was reported in a clinical trial of early detection [
7]. However, conduct of further studies might be needed in order to evaluate the effectiveness of liver cancer screening with a final outcome indicator such as LYS, QALY, or DALY.
Despite these limitations, to the best of our knowledge, this is the first study to use a probability model for identification of cost-effective liver cancer screening strategies. The analysis used to simulate the estimated cost of liver cancer screening was based on fewer assumptions and provided more robust results when compared to the existing cost-effectiveness analysis method.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download