Abstract
Cases of phenotypic heterogeneity of cells within tumors have recently been reported. Here, we report on a patient with characteristic intra-tumor double primary metastases in the lung. This patient was a 40-year-old Korean woman who had been diagnosed with breast cancer (T1N0M0, estrogen receptor/progesterone receptor/HER2 +/+/+) and papillary thyroid cancer three years prior and underwent a complete surgical resection followed by appropriate adjuvant treatment with radiation, hormone, and radioactive iodine. She was recently admitted for newly developed pulmonary nodules. Metastasectomy through video-assisted thoracoscopic surgery revealed recurrent double primary cancer with two different components (metastatic ductal carcinomas from the breast and metastatic papillary carcinomas from the thyroid gland) in each pulmonary nodule in the right upper lobe and right middle lobe. To the best of our knowledge, this is the first report of simultaneous recurrent double metastasis in one organ from different primary origins.
Cancer cells have the ability not only to grow in the tissue of origin, but also to propagate and colonize distant sites. This is known as metastasis, and metastasis to an organ or another tumor is related to anatomic location, vascularity, and the local immune response to the tumor [1,2]. Metastasis is the main cause of failure of cancer treatment, since undiscovered micrometastases can remain even after complete resection of the primary tumor [3,4]. Solid tumors of different origin are occasionally found to share the same metastatic signature [5].
With the increase in the average human lifespan and development of better techniques for diagnosis of cancer, the number of reports of double primary cancers has increased [6,7,8]. The prognosis of double primary cancers is dependent on the availability of treatments with curative aims. If one cancer overrides the other malignancy, active and aggressive treatment for the latter might result in inefficacious treatment. Occasionally, it is unclear which treatment modality is correct for a specific clinical setting. Metastasis is a process by which cancer cells endure and survive. Simultaneous metastasis of two clones of different origins to one organ is rare. In this regard, the current case may provide additional understanding of the metastatic cascade. Here, we report on a case of simultaneous recurrent double metastases in one organ from different primary origins despite resection of the primary sites. To the best of our knowledge, no case of double metastatic cancer tissues presenting in the same pulmonary nodule has been reported. We describe a patient with recurrent double primary metastatic cancers (metastatic ductal carcinoma from the breast and metastatic papillary carcinoma from the thyroid gland) found simultaneously in pulmonary nodules on two lobes of the right lung (the right upper lobe [RUL] and right middle lobe [RML]).
A 40-year-old Korean woman was admitted to Samsung Medical Center for newly developed pulmonary nodules. The patient had no family history of malignancy. Three years prior, she had been diagnosed with cancer of the left breast and underwent left breast-conserving surgery with sentinel lymph node biopsy. Postoperative pathologic findings showed invasive ductal carcinoma and indicated that she was positive for both estrogen receptor (ER) and progesterone receptor. HER2 immunohistochemistry was 3+. The TNM stage was 1 (T1N0M0). At the same time, she was also diagnosed with papillary thyroid cancer (diffuse sclerosing variant [DSV], bilateral lobes) and underwent a total thyroidectomy with modified radical neck dissection. Since then, she has received radiation therapy for breast cancer and was given tamoxifen and goserelin as adjuvant therapy. Radioactive iodine therapy (50 mci×3) was administered three times for the thyroid cancer.
Her chest computed tomography (CT) scan showed at least three well-defined nodules in the right upper and middle lobes, suggesting pulmonary metastases (Fig. 1). She underwent wedge resection of the RUL and RML using video-assisted thoracotomy for metastasectomy and a definitive diagnosis at the department of thoracic surgery. A nodule on the RUL and two nodules on the RML observed by chest CT scan were found operatively. The pathologic diagnosis was recurrent double primary cancer in both lobes.
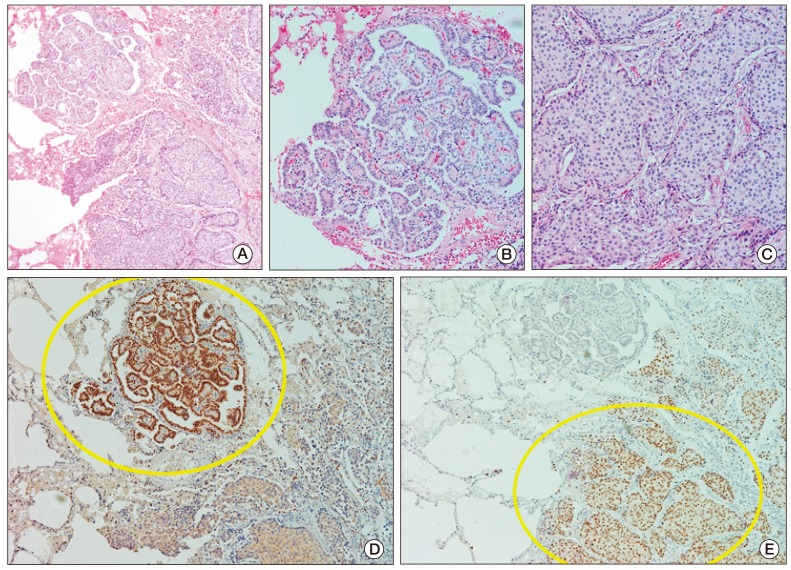
There were two different components (metastatic ductal carcinoma from the breast and metastatic papillary carcinoma from the thyroid gland) in one pulmonary nodule in the RUL and RML. In the RML, a metastatic ductal carcinoma measuring 0.4 cm in size from the breast and metastatic papillary carcinoma measuring 0.15 cm in size were found. In the RUL, a metastatic ductal carcinoma measuring 0.9 cm in size from the breast and metastatic papillary carcinoma measuring 0.1 cm were found. We confirmed this diagnosis through thyroid transcription factor-1 (TTF-1) and ER immunohistochemical staining (Fig. 2). There were two components on each slide. In the TTF-1 stain, we observed positive signals in the papillary carcinoma component. The other tumor components showed ER-positive signals from the ductal carcinoma of the breast (Fig. 2). Intratumoral double primary carcinomas were found together in the three resected metastatic tumors.
Since then, the patient received six cycles of docetaxel plus trastuzumab combination chemotherapy and additional radioactive iodine therapy. She is currently under treatment with trastuzumab and tamoxifen with stable disease.
This case had a unique clinicopathological presentation, with several points of interest in terms of metastasis. First, this case is an extreme example of intra-tumor heterogeneity, and clinicopathologic evidence of intratumoral metastases from completely different, synchronous clones was found. Cases of phenotypic heterogeneity of cells within tumors have recently been reported [9,10]. According to these reports, intra-tumor heterogeneity may result from genetic diversity due to clonal evolution or multiple cancer stem cells. In addition, according to the classic model of human cancer development, transcription factors involved in promotion of epithelial-to-mesenchymal transition (EMT) are activated in some cancer cells residing at the invasive edge of advanced cancers. According to this model, activation of proteins associated with EMT does not provide a growth advantage within the primary tumor, but rather mediates the final step in tumor progression. However, the results of some recent studies have supported a different model, which predicts that expression of proteins that mediate the EMT promotes malignant conversion concomitantly with metastatic dissemination [11,12]. In other words, tumor progression and metastasis are a result of early dissemination from original tumor cells with metastatic potential, even at the premalignant stage, rather than being the result of selection of a subpopulation of cells evolving within the primary tumors. However, these results were obtained from single primary cancers, not different double primary malignancies. The occurrence of synchronous double primary cancers in one tumor might be caused by a specific microenvironment in the host, either genetic or non-genetic. Presumably, this patient might have complex, underlying genetic alterations that have yet to be defined.
Second, and most importantly, this case strongly highlights the value of repeated biopsy when metastases are documented. In this case, metastasectomy was performed because we could not confirm which tumor had progressed to the lung, not because double primary metastatic tumors in the same tumor were suspected. Phenotypic heterogeneity can sometimes be observed between the primary and metastatic sites of a single primary tumor. This feature is important both for prognosis and treatment. If a biopsy had not been performed, the further treatment modalities chosen might have been unsuitable.
Finally, both HER2-overexpressing breast cancer and DSV can be aggressive, with cancer progression and metastasis. In addition, the lung is the most common organ of metastasis from breast and thyroid carcinomas. Solid tumors of different origins showing the same metastatic signature were found, suggesting involvement of a common set of molecules in regulation of metastasis in a variety of primary tumors. Expression of lung metastasis gene-expression signature can predict the risk of lung metastasis and prognosis, particularly in primary breast cancers, which was validated by two large cohorts of patients with early stage breast cancer [13,14,15].
We experienced and diagnosed a patient with characteristic intra-tumor double primary metastases in the lung consisting of ductal carcinomas from the breast and papillary carcinomas from the thyroid.
References
1. Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997; 80(8 Suppl):1529–1537. PMID: 9362419.

2. Gonullu G, Sullu Y, Basoglu A, Elmali M, Karaoglanoglu M, Yucel I. Metastatic breast carcinoma to solitary fibrous tumor in the lung. Indian J Cancer. 2010; 47:76–78. PMID: 20071799.

3. Riethmuller G, Klein CA. Early cancer cell dissemination and late metastatic relapse: clinical reflections and biological approaches to the dormancy problem in patients. Semin Cancer Biol. 2001; 11:307–311. PMID: 11513566.
4. Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002; 12:89–96. PMID: 12027580.

5. Pedraza-Farina LG. Mechanisms of oncogenic cooperation in cancer initiation and metastasis. Yale J Biol Med. 2006; 79:95–103. PMID: 17940619.
6. Choi KK, Choi SB, Park SW, Kim HK, Park YN, Kim KS. Synchronous double primary cancers associated with a choledochal cyst and anomalous pancreaticobiliary ductal union. J Korean Surg Soc. 2011; 81:281–286. PMID: 22111085.

7. Kim YW, Ryu H, Kim DS, Kim IY. Double primary malignancies associated with colon cancer in patients with situs inversus totalis: two case reports. World J Surg Oncol. 2011; 9:109. PMID: 21943483.

8. Lin CC, Chian CF, Perng WC, Cheng MF. Synchronous double primary lung cancers via p53 pathway induced by heavy smoking. Ann Saudi Med. 2010; 30:236–238. PMID: 20427942.

9. Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012; 12:323–334. PMID: 22513401.

10. Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012; 366:883–892. PMID: 22397650.

11. Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008; 454:49–55. PMID: 18509334.

12. Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008; 14:79–89. PMID: 18598946.

13. Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003; 33:49–54. PMID: 12469122.

14. van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002; 347:1999–2009. PMID: 12490681.

15. Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005; 365:671–679. PMID: 15721472.

Fig. 1
Chest non-contrast computed tomography (CT) scan. CT scans were obtained using a non-contrast helical CT scan protocol. Three small, well-defined nodules were found in the right upper and middle lobes of the lung, suggesting pulmonary metastases.
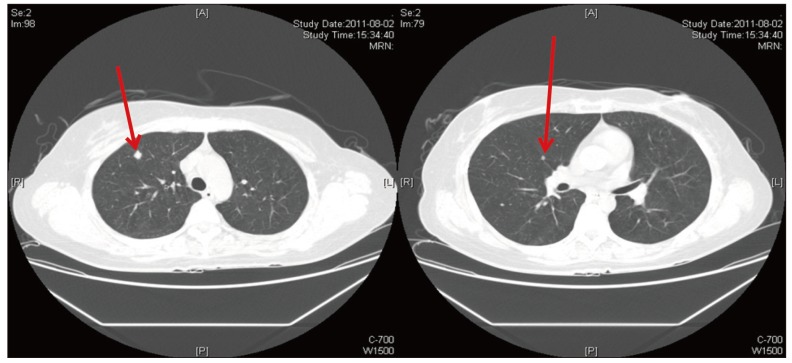
Fig. 2
Pathologic findings in the right middle lobe of the lung. (A) Ductal carcinoma and papillary carcinoma (H&E staining, ×100). (B) Papillary carcinoma (H&E staining, ×400). (C) Ductal carcinoma (H&E staining, ×400). (D) Thyroid transcription factor-1 immunohistochemistry (IHC) stain (uptake only in papillary carcinoma; yellow circular line) (×100). (E) Estrogen receptor IHC stain (uptake only in ductal carcinoma; yellow circular line) (×100).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download