Abstract
Purpose
This study was conducted to evaluate the efficacy and safety of azasetron compared to ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting.
Materials and Methods
This study was a multi-center, prospective, randomized, double-dummy, double-blind and parallel-group trial involving 12 institutions in Korea between May 2005 and December 2005. A total of 265 patients with moderately and highly emetogenic chemotherapy were included and randomly assigned to either the azasetron or ondansetron group. All patients received azasetron (10 mg intravenously) and dexamethasone (20 mg intravenously) on day 1 and dexamethasone (4 mg orally every 12 hours) on days 2-4. The azasetron group received azasetron (10 mg orally) with placebo of ondansetron (orally every 12 hours), and the ondansetron group received ondansetron (8 mg orally every 12 hours) with placebo of azasetron (orally) on days 2-6.
Results
Over days 2-6, the effective ratio of complete response in the azasetron and ondansetron groups was 45% and 54.5%, respectively (95% confidence interval, -21.4 to 2.5%). Thus, the non-inferiority of azasetron compared with ondansetron in delayed chemotherapy-induced nausea and vomiting was not proven in the present study. All treatments were well tolerated and no unexpected drug-related adverse events were reported. The most common adverse events related to the treatment were constipation and hiccups, and there were no differences in the overall incidence of adverse events.
Go to : 
Chemotherapy-induced nausea and vomiting (CINV) is a significant concern for cancer patients undergoing chemotherapy and can result in various metabolic and nutritional problems and sometimes even discontinuation of chemotherapy [1]. CINV is classified as anticipatory, acute (within 24 hours after the initiation of chemotherapy), and delayed emesis (2-5 days later). The 5-hydroxytryptamine-3 (5-HT3) receptor antagonists have exhibited efficacy and safety in the prevention of CINV, as well as postoperative and radiotherapy-induced nausea and vomiting [2-4]. Azasetron, a selective potent 5-HT3 receptor antagonist, is a derivative of benzamide with a different chemical structure from other 5-HT3 receptor antagonists, such as ondansetron, granisetron, ramosetron, and tropisetron, and has a longer duration of action and a higher affinity for the 5-HT3 receptor [5,6]. Azasetron has shown efficacy and safety in the prevention of CINV and postoperative nausea and vomiting, while that in delayed CINV has not been confirmed in a prospective randomized controlled trial [3,7,8]. Therefore, the present study was conducted to evaluate the efficacy and safety of azasetron compared with ondansetron for the prevention of delayed CINV.
Go to : 
Patients were recruited for this trial from 12 institutions in Korea between May 2005 and December 2005. Eligibility criteria were men and women aged between 19 and 75 with confirmed malignancy, who planned to receive moderately or highly emetogenic chemotherapeutic drug (Hesketh level 3-5) on day 1, and who were naive to chemotherapy or had previous chemotherapy≥3 weeks prior to screening [9]. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status 1-2, a life expectancy≥3 months, and adequate hepatic and renal function (aspartate aminotransferase and alanine aminotransferase≤2.5×upper normal limit [UNL], bilirubin ≤1.5×UNL, and creatinine≤1.5×UNL).
Exclusion criteria were as follows: severe uncontrolled concurrent illness other than malignancy; other causes of nausea and vomiting (hypercalcemia, intestinal obstruction); current medications for active peptic ulcer; metastatic or primary tumor in the central nervous system (CNS); any nausea or vomiting within 24 hours before chemotherapy; grade 3 or higher nausea or vomiting according to Common Terminology Criteria of Adverse Event, ver. 3.0 (CTCAE V.3) in previous chemotherapies within 6 months; a known hypersensitivity to 5-HT3 receptor antagonists or dexamethasone; medications that may affect the gastrointestinal (GI) tract or CNS in the previous 48 hours; radiotherapy within 3 weeks or unstable state after surgery; and participation in another drug study.
All patients were randomly assigned to one of the two treatment groups before chemotherapy and instructed to take a daily dose of antiemetics according to the regimens listed in Table 1. Patients who developed nausea or vomiting requiring rescue therapy discontinued the study and were managed appropriately in each institution.
Treatment regimens
The study was approved by the ethics committee of each institution and all patients gave written informed consent.
Patients recorded episodes of nausea and vomiting, and visual analogue scale (VAS) in self diaries for 6 days (days 1-6). Patients visited the hospital on day 7 (+2) and week 4 (±1 week), and laboratory examinations (complete blood cell count [CBC], blood chemistry [BC], and urinalysis [UA]), electrocardiogram (ECG), and physical examinations including vital signs were checked.
The primary end point was the effective ratio of complete response (CR) for CINV (ratio of CR on days 2-6 to during 24 hours). The secondary end points were degree of nausea by treatment day (grade 0-3), complete control (CC, CR of vomiting and nausea of grade 0-1) by treatment day, physician's global assessment (PGA) on day 7 (absent, very mild, mild, moderate, severe), and VAS by treatment day.
Adverse events (AEs) were graded according to CTCAE V.3, and the relationship to the study treatments was assessed by the investigators.
The intention-to-treat (ITT) set included all randomized patients who received the study drug≥1 time, and the safety set included all randomized patients who received the study drug≥1 time and in whom the safety profile was checked≥1 time. The baseline data and efficacy results are based on the ITT set and the safety results on the safety set.
Chi-square test or t-test was used to compare baseline data. The primary efficacy postulation was that azasetron was not inferior to ondansetron in the control of delayed CINV (days 2-6) using the estimated lower boundary of the 95% confidence interval (CI) in comparison with the predefined non-inferiority margin of -15%. The degree of nausea, CC, and VAS by treatment day were compared between the two groups using t-test, PGA using chi-square test or Fisher's exact test, and safety was compared using Fisher's exact test. Statistical significance was set at two-sided; p<0.05. All statistical analyses were done by use of the SAS ver. 8.1 (SPSS Inc., Chicago, IL).
Go to : 
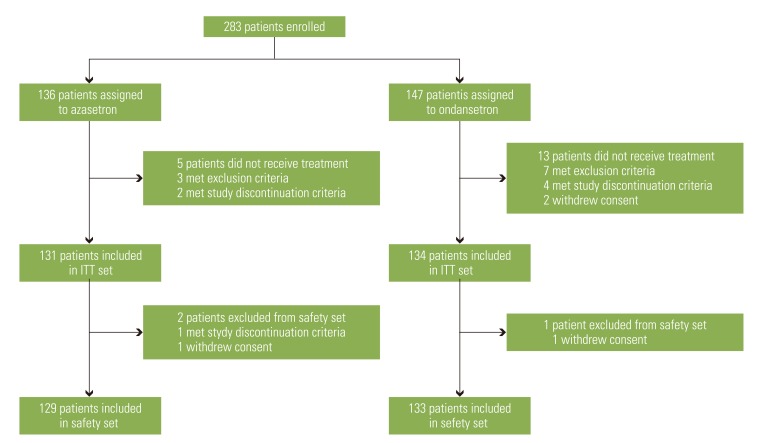
A total of 283 patients enrolled in the study, but 18 patients did not receive the treatment; thus 265 patients were randomized (131 in the azasetron group and 134 in the ondansetron group). Three patients were excluded from the safety set, so safety was assessed in 262 patients (Fig. 1).
Patient characteristics are summarized in Table 2. There were no significant differences between the two groups in terms of sex, age, ECOG performance status, emetogenicity, alcohol intake, and previous and current medical history including previous chemotherapy and current anti-cancer treatments. In laboratory examinations (CBC, BC, and UA), ECG, and physical examinations, no significant differences were identified between the two groups.
Patient characteristics
The primary end point was the effective ratio of CR. Table 3 illustrates the effective ratio of CR: 45% in the azasetron group; 54.5% in ondansetron (95% CI, -21.4 to 2.5%). The estimated lower boundary of the 95% CI was -21.4%, which was lower than the predefined non-inferiority margin of -15%; thus, the non-inferiority of azasetron compared to ondansetron in the control of CINV was not validated in the current study. Subgroup analysis for the effective ratio of CR according to previous chemotherapy (naïve and non-naïve), sex, and institution was conducted and the results did not confirm the non-inferiority of azasetron (Table 3).
Effective ratio of complete response (CR)
The results of secondary end points (degree of nausea; CC; PGA on day 7; and VAS) are summarized in Tables 4-6. Although the degree of nausea and the CC by treatment day were not significantly different between the two groups, the azasetron group showed trends of higher degree of nausea than ondansetron (Table 4). The visit on day 7 was missed by 21 patients, so PGA assessment was available in 244 patients. The PGA on day 7 and VAS were not significantly different between the two groups, while more patients were graded 'very mild' on PGA in the ondansetron group than in the azasetron group (Tables 5 and 6).
Degree of nausea and complete control by treatment day
Azasetron and ondansetron showed similar safety profiles in regards to total, treatment-related, and serious AEs. Treatment-related AEs are summarized in Table 7. Constipation and hiccups were more common in the azasetron group (6.2%, each) and hiccups followed by constipation in the ondansetron group (10.6% and 6.8%, respectively). Additionally, physical examinations including vital signs and laboratory examinations on visiting days (day 7 and week 4) were not different between the two groups.
Treatment-related adverse events developed in≥2% of patients in each group in the safety set (n=262)
Go to : 
The present study aimed to evaluate the efficacy and the safety of azasetron compared to ondansetron in the prevention of delayed CINV. The current study showed inferiority of azasetron in the prevention of delayed CINV (the effective ratio of CR) compared to ondansetron, while the degree of nausea, CC, and VAS by treatment day and safety profile were similar between the two groups. In this respect, the little CNS distribution of azasetron compared to ondansetron and the possibility of inadequate dosing schedule should be considered. Previous studies have revealed that azasetron showed far less brain distribution and little correlation between blood and brain, whereas ondansetron exhibited good correlation between plasma and cerebrospinal fluid [10-12]. The mechanisms of delayed CINV have not been well defined; however, the concept that CINV is primarily mediated by neurotransmitter in the GI tract and the CNS has been widely accepted. The 5-HT3 receptor is located in both the GI tract and the CNS, while the 5-HT3 receptor antagonists predominantly act on peripheral sites, which is well illustrated in animal models and accounts for the ineffectiveness of these drugs in delayed CINV [4,13,14]. Additionally, the antiemetic duration of action of azasetron compared to ondansetron was not much different in a preclinical study, and a study of dosage regimens revealed that the divided (twice) and continuous infusion regimens are more effective than the bolus (once) regimen, thus the once daily schedule of azasetron could have been insufficient in comparison with the twice daily schedule of ondansetron [15,16].
In acute CINV, 5-HT3 receptor antagonists including azasetron and ondansetron have shown efficacy and safety and are a current standard in combination with dexamethasone±aprepitant [17-20]. However, the pathophysiology of delayed CINV and the role of 5-HT3 receptor antagonists have remained elusive and the aforementioned lack of central effects of these drugs has been accepted [4,14]. Three large-scale studies conducted by the National Cancer Institute of Canada Clinical Trials Group and the Italian Group for Antiemetic Research concluded that adding a 5-HT3 receptor antagonist to dexamethasone beyond 24 hours after chemotherapy has no significant benefit in the control of delayed CINV [21-23]. Additionally, neurokinin-1 (NK1) receptor antagonists have shown superiority to 5-HT3 receptor antagonists in delayed CINV and thus are a current standard in combination with dexamethasone. In contrast to 5-HT3, substance P binds to NK1 receptor mainly in the medulla and triggers emesis. Substance P also binds to receptors in the gut, but has an accessory role in CINV [24]. Moreover, NK1 receptor antagonists (aprepitant, fosaprepitant) penetrate the blood brain barrier and are retained in brain tissues more than 48 hours in an animal study [25]. Therefore, further studies with 5-HT3 receptor antagonist in the delayed CINV do not seem to be worthwhile.
Go to : 
The present study showed the inferiority of azasetron in the prevention of delayed CINV in comparison with ondansetron. However, azasetron and ondansetron have similar safety profiles and, in regards to acute CINV, the efficacy and safety of azasetron are well known. The pathophysiology of delayed CINV and the role of 5-HT3 receptor antagonists are unclear. Additionally, NK1 receptor antagonists are a current standard due to their superiority over 5-HT3 receptor antagonists. Thus, further studies of the 5-HT3 receptor antagonists in terms of delayed CINV do not seem to be necessary.
Go to : 
References
1. Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer. 2005; 13:219–227. PMID: 15538640.

2. Cheirsilpa A, Sinthusake T, Songsakkaesorn A, Visawaprasit S, Chulaka K, Changkuingdee N. Comparison of ramosetron and granisetron for the prevention of acute and delayed emesis in cisplatin-based chemotherapy: a randomized controlled trial. Jpn J Clin Oncol. 2005; 35:695–699. PMID: 16319109.

3. Yun MJ, Kim YH, Kim AR. Comparison of azasetron and ondansetron for preventing postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery. Yonsei Med J. 2010; 51:88–92. PMID: 20046519.

4. Navari RM. Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting: two new agents. J Support Oncol. 2003; 1:89–103. PMID: 15352652.
5. Sakamori M, Takehara S, Setoguchi M. High affinity binding of Y-25130 for serotonin 3 receptor. Nihon Yakurigaku Zasshi. 1992; 100:137–142. PMID: 1330854.

6. Tsukagoshi S. Pharmacokinetics of azasetron (Serotone), a selective 5-HT3 receptor antagonist. Gan To Kagaku Ryoho. 1999; 26:1001–1008. PMID: 10396331.
7. Hayakawa T, Sato M, Konaka M, Makino A, Hirohata T, Totsu S, et al. Comparison of ramosetron and azasetron for prevention of acute and delayed cisplatin-induced emesis in lung cancer patients. Gan To Kagaku Ryoho. 2006; 33:633–638. PMID: 16685162.
8. Kimura E, Niimi S, Watanabe A, Tanaka T. Clinical effect of two azasetron treatment methods against nausea and vomiting induced by anticancer drugs including CDDP. Gan To Kagaku Ryoho. 1997; 24:855–859. PMID: 9170525.
9. Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997; 15:103–109. PMID: 8996130.

10. Ishiwata K, Ishii K, Ishii S, Senda M. Synthesis of 5-HT3 receptor antagonists, [11C] Y-25130 and [11C] YM060. Appl Radiat Isot. 1995; 46:907–910. PMID: 7581293.
11. Yang SH, Lee MG. Dose-independent pharmacokinetics of ondansetron in rats: contribution of hepatic and intestinal first-pass effects to low bioavailability. Biopharm Drug Dispos. 2008; 29:414–426. PMID: 18697186.

12. Simpson KH, Murphy P, Colthup PV, Whelan P. Concentration of ondansetron in cerebrospinal fluid following oral dosing in volunteers. Psychopharmacology (Berl). 1992; 109:497–498. PMID: 1365869.

13. Rudd JA, Naylor RJ. Effects of 5-HT3 receptor antagonists on models of acute and delayed emesis induced by cisplatin in the ferret. Neuropharmacology. 1994; 33:1607–1608. PMID: 7760983.
14. Geling O, Eichler HG. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005; 23:1289–1294. PMID: 15718327.

15. Haga K, Inaba K, Shoji H, Morimoto Y, Fukuda T, Setoguchi M. The effects of orally administered Y-25130, a selective serotonin3-receptor antagonist, on chemotherapeutic agent-induced emesis. Jpn J Pharmacol. 1993; 63:377–383. PMID: 8107329.

16. Yamada Y, Fujita M, Okuyama K, Takayanagi R, Ozeki T, Yokoyama H, et al. Analysis of antiemetic effect of various dosage regimens of azasetron hydrochloride based on 5-HT3 receptor occupancy of serotonin. Yakugaku Zasshi. 2007; 127:353–357. PMID: 17268155.

17. Perez EA. Review of the preclinical pharmacology and comparative efficacy of 5-hydroxytryptamine-3 receptor antagonists for chemotherapy-induced emesis. J Clin Oncol. 1995; 13:1036–1043. PMID: 7707101.

18. Tsukuda M, Mochimatsu I, Furukawa M, Kohno H, Kawai S, Enomoto H, et al. A randomized crossover comparison of azasetron and granisetron in the prophylaxis of emesis induced by chemotherapy including cisplatin. Gan To Kagaku Ryoho. 1995; 22:1959–1967. PMID: 7487127.
19. Kimura E, Niimi S, Watanabe A, Akiyama M, Tanaka T. Study on clinical effect of a continuous intravenous infusion of azasetron against nausea and vomiting induced by anticancer drugs including CDDP. Gan To Kagaku Ryoho. 1996; 23:477–481. PMID: 8678501.
20. Navari R, Gandara D, Hesketh P, Hall S, Mailliard J, Ritter H, et al. The Granisetron Study Group. Comparative clinical trial of granisetron and ondansetron in the prophylaxis of cisplatin-induced emesis. J Clin Oncol. 1995; 13:1242–1248. PMID: 7738628.
21. Latreille J, Pater J, Johnston D, Laberge F, Stewart D, Rusthoven J, et al. National Cancer Institute of Canada Clinical Trials Group. Use of dexamethasone and granisetron in the control of delayed emesis for patients who receive highly emetogenic chemotherapy. J Clin Oncol. 1998; 16:1174–1178. PMID: 9508205.
22. Pater JL, Lofters WS, Zee B, Dempsey E, Walde D, Moquin JP, et al. The role of the 5-HT3 antagonists ondansetron and dolasetron in the control of delayed onset nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Ann Oncol. 1997; 8:181–185. PMID: 9093728.
23. The Italian Group for Antiemetic Research. Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med. 2000; 342:1554–1559. PMID: 10824073.
24. Diemunsch P, Grelot L. Potential of substance P antagonists as antiemetics. Drugs. 2000; 60:533–546. PMID: 11030465.

25. Huskey SE, Dean BJ, Bakhtiar R, Sanchez RI, Tattersall FD, Rycroft W, et al. Brain penetration of aprepitant, a substance P receptor antagonist, in ferrets. Drug Metab Dispos. 2003; 31:785–791. PMID: 12756213.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download