Abstract
Purpose
Evidence regarding the usefulness of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in predicting the prognosis of non-small cell lung cancer is increasing. However, data on small cell lung cancer (SCLC) are scarce. The aim of this study was to evaluate the prognostic value of metabolic parameters measured using 18F-FDG PET/CT in patients with SCLC.
Materials and Methods
We conducted a retrospective review of 114 patients with pathologically proven SCLC (26 cases of limited disease and 88 cases of extensive disease) who underwent pretreatment 18F-FDG PET/CT. The maximal SUV (SUVmax) was used quantitatively for determination of FDG PET activity. The SUVmax of the primary tumor (primary SUVmax), the sum of SUVmax values of malignant lesions (SUVsum), and the mean SUVmax of malignant lesions were calculated.
Results
The patient population was subdivided using a median SUVsum value of 24.6. High SUVsum showed a significant association with known factors for poor prognosis, including higher neuron-specific enolase (p=0.010), CYFRA 21-1 (p=0.014), and extensive disease status (p=0.007). Patients with high SUVsum had significantly shorter median overall survival (6.6 months vs. 13.0 months, p<0.001) and progression-free survival (5.2 months vs. 8.0 months, p<0.001) than patients with low SUVsum. Results of multivariate analysis showed that SUVsum, chemotherapy cycles, and the response to first-line treatment were significant prognostic factors of survival. In contrast, mean SUVmax and primary SUVmax were not significant predictors of survival.
Lung cancer is the leading cause of cancer-related deaths worldwide. Small cell lung cancer (SCLC) represents 15-20% of all lung cancers [1]. Despite the use of more active and intensive regimens, the survival time of SCLC patients has shown only modest improvement over the past two decades due to the aggressive nature of the disease [2].
SCLC is divided into two stages: limited disease (LD), disease confined to one hemithorax, which can be encompassed in a tolerable radiation field, and extensive disease (ED), which includes spread of the tumor beyond these manifestations [3]. Currently, tumor stage is the most important prognostic factor in SCLC: other prognostic factors include performance status, weight loss, sex, serum albumin, and serum lactate dehydrogenase (LDH) levels [4, 5]. Despite its practical usefulness and prognostic advantages, the two-stage system is not accurate enough to reflect tumor burden and is inadequate for predicting survival in some patients. Therefore, prognostic factors with greater discriminatory power are needed for stratification of risk groups with the goal of developing individualized therapeutic strategies.
Positron emission tomography (PET) or fused positron emission tomography/computed tomography (PET/CT) imaging using the tracer 18F-fludrodeoxyglucose (18F-FDG) has been established as a standard technique for staging various cancers, including lung cancer. The degree of tumor uptake of 18F-FDG on PET/CT, as measured based on standardized uptake value (SUV), was recently shown to be an important prognostic factor in lung cancer and other malignant tumors [6, 7, 8, 9]. However, it remains uncertain whether 18F-FDG PET/CT can provide prognostic information for patients with SCLC. To date, only a few studies have addressed the prognostic role of 18F-FDG uptake in patients with SCLC [10,11].
In the current study, we investigated the prognostic value of metabolic parameters of pretreatment 18F-FDG PET/CT in patients with SCLC.
We retrospectively reviewed the tumor registry at our institution and identified all patients diagnosed with SCLC between January 2005 and February 2012. Patients who met the following inclusion criteria were selected for the current study: 1) histologically or cytologically confirmed diagnosis of primary SCLC; 2) pretreatment 18F-FDG PET/CT scanning; 3) adequate clinical data recorded in medical charts; and 4) treatment with at least one cycle of chemotherapy. Finally, we enrolled 114 consecutive patients. This study was approved by the institutional review board of our institution.
Patients underwent combined chemotherapy with etoposide, cisplatin (EP) or irinotecan, cisplatin (IP) regimen as the first-line treatment. The EP regimen consisted of etoposide 120 mg/m2 on days 1-3 and cisplatin 60 mg/m2 on day 1. The IP regimen consisted of irinotecan 60 mg/m2 on days 1, 8, and 15 and cisplatin 60 mg/m2 on day 1. Both regimens were repeated with an interval of three weeks.
The Response Evaluation Criteria in Solid Tumor ver. 1.1 (RECIST 1.1) was used in assessment of the treatment response via a CT scan acquired every two or three cycles; the responses were classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Objective response included both CR and PR.
18F-FDG PET/CT imaging was performed using a dedicated PET/CT scanner (Gemini, Philips, Milpitas, CA) consisting of a dedicated germanium oxyorthosilicate full-ring PET scanner and a dual slice helical CT scanner. Standard patient preparation included at least 8 hours of fasting and a serum glucose level of less than 140 mg/dL before administration of 18F-FDG. Two experienced nuclear medicine physicians who were unaware of the patients' clinical information were responsible for reading the 18F-FDG PET/CT images. The maximal SUV (SUVmax) was used quantitatively for determination of FDG PET activity. SUVmax was defined as the peak SUV in one pixel with the highest counts within the region of interest. The highest SUVmax of the primary lesion (primary SUVmax), calculated as the mean of the highest SUVmax values of malignant lesion for each organ (mean SUVmax), and the sum of the highest SUVmax values of malignant lesion for each organ (SUVsum) were selected as PET/CT parameters.
Overall survival (OS) and progression-free survival (PFS) were chosen as endpoints for evaluation of the prognostic value of 18F-FDG PET/CT. OS was defined as the time interval from the date of 18F-FDG PET/CT scanning to the date of death from any cause, or last follow-up. PFS was defined as the time interval from the date of 18F-FDG PET/CT scanning to the date of first progression or death from any cause without previous progression. The Kaplan-Meier method was used for estimation of survival time and the log-rank test was used for assessment of difference in survival between groups The Cox proportional hazards model was used for multivariate analysis of survival. Probability values <0.05 were considered statistically significant. SPSS ver. 18.0 (SPSS Inc., Chicago, IL) was used for statistical analyses.
A total of 114 eligible patients were enrolled in the study. A summary of the patients' baseline characteristics is shown in Table 1. The median age of the patients was 67 years (range, 41 to 86 years) and 105 (92.1%) patients were male. The median number of cycles of chemotherapy was six (range, 1 to 12). Follow-up data were available through August 2012. At the time of analysis, 15 (13.2%) patients were still alive, with a median follow-up period of 10.5 months (range, 1.2 to 43.9 months). The estimated median OS and PFS for the entire cohort was 10.6 months (95% confidence interval [CI], 8.4 to 12.8 months) and 6.6 months (95% CI, 5.5 to 7.7 months), respectively.
Tumors were detected by 18F-FDG PET/CT scanning in all patients. The median number of target organs per patient that showed metabolic activity was three (range, 1 to 8).
Three variables based on the SUVmax were evaluated: SUVsum (median, 24.6; 95% CI, 35.4 to 53.7), mean SUVmax (median, 6.4; 95% CI, 6.7 to 7.9), and primary SUVmax (median, 8.3; 95% CI, 8.3 to 9.9). The study population was divided into two groups according to a median SUVsum of 24.6. A list of descriptive statistics for the two groups is shown in Table 1. Patients with high SUVsumwere more likely to have higher levels of neuron-specific enolase (NSE) (p=0.010) and CYFRA 21-1 (p=0.014) and to have ED status (p=0.007) compared to patients with low SUVsum (Table 1). The patients were also divided into two groups based on the median primary SUVmax and mean SUVmax; however, no significant difference was observed between these two groups (data not shown).
The high-SUVsum group showed significantly shorter median OS (6.6 months vs. 13.0 months, p<0.001) and PFS (5.2 months vs. 8.0 months, p<0.001) than the low-SUVsum group (Fig. 1). Results of univariate analysis of OS showed that sex, performance status, primary SUVmax, and mean SUVmax were not significant predictors of OS, whereas SUVsum, age, LDH, NSE, CYFRA 21-1 levels, tumor stage, chemotherapy cycles, and response to first-line treatment were significant predictors of OS. In multivariate analysis, SUVsum, age, chemotherapy cycles, and response to first-line treatment remained significant predictors of OS (Table 2).
A result similar to that observed for OS was obtained for the association between PFS and these factors. Results of univariate analysis showed that age, performance status, primary SUVmax, and mean SUVmax were not significant predictors of PFS, whereas SUVsum, sex, tumor stage, chemotherapy cycles, and response to first-line treatment were significant predictors of PFS. Results of multivariate analysis showed that SUVsum, sex, chemotherapy cycles, and response to first-line treatment were independent predictors of PFS (Table 2).
A strong association between SUVsum and PFS was also found within each disease stage. Patients with LD and high SUVsum (n=7) showed a significantly shorter median PFS (7.7 months vs. 9.8 months, p=0.001) than patients with LD and low SUVsum (n=19) (Fig. 2A). Patients with ED and high SUVsum (n=50) also had a significantly shorter median PFS (5.0 months vs. 7.4 months, p=0.002) than patients with the same stage and low SUVsum (n=38) (Fig. 2A). Similar results were obtained regarding OS. Patients with ED and high SUVsum showed a significantly shorter median OS (6.6 months vs. 11.0 months, p<0.001) than patients with ED and low SUVsum (Fig. 2B). However, no significant association between OS and SUVsum was observed in patients with LD (p=0.703) (Fig. 2B). Of particular interest, patients with LD and high SUVsum had similar median OS (11.7 months vs. 11.0 months, p=0.602) and PFS (7.7 months vs. 7.4 months, p=0.537) compared to patients with ED and low SUVsum (Fig. 2).
Table 3 shows the distribution of the best responses to first-line treatment. A total of 114 patients received chemotherapy and 91 patients were evaluated. An objective response rate was achieved in 41.2% of all treated patients. Regarding the objective responses to treatment, a significant difference was observed between the high-SUVsum group and the low-SUVsum group (19.0% vs. 40.8%, p=0.025).
Results of this study showed a strong association of SUVsum, the sum of the SUVmax values of 18F-FDG PET/CT, with survival in SCLC. Patients with low pretreatment SUVsum had significantly longer OS and PFS. In contrast, mean SUVmax and primary SUVmax were not significant predictors of OS and PFS inpatients with SCLC.
18F-FDG PET/CT has become an essential imaging tool for management of lung cancer. SUV is the PET parameter most commonly used as a semiquantitative index of 18F-FDG PET/CT. This parameter reflects tumor glucose metabolism and is represented by the mean (SUVmean) value or by the maximum (SUVmax) value [12]. Previous studies have suggested an association between SUVmax and tumor aggressiveness [7,10,13]. However, measurement of SUVmax was confined to detection of the most evident metabolic activity of the tumor at a single site, and not of the overall tumor activity. In general, SCLC shows a rapid doubling time, a high growth fraction, and early development of widespread metastases [14]. Therefore, SUVmax at a single site or the mean value of SUVmax is not sufficiently accurate to reflect real tumor metabolic activity in an aggressive cancer, such as SCLC. In the current study, in order to compensate for this shortcoming, SUVsum was used as an indicator that potentially reflects the overall tumor metabolic burden of the entire body.
The results obtained here are consistent with those of Zhu et al. [11], who reported that metabolic tumor volume (MTV), a volumetric parameter of 18F-FDG PET/CT, is an important independent prognostic factor in patients with SCLC. MTV represents the amount of highly metabolic tumor cells [11]. There is a thread of connections showing that the parameters reflecting the entire tumor metabolic burden, such as SUVsum or MTV, are more effective predictors of survival in patients with SCLC than SUVmax at a single site or mean SUVmax. However, calculation of MTV is more complicated than that of SUVsum because it generally requires use of additional commercial software. Thus, we cautiously suggest that clinicians should focus on the sum of SUVmax value rather than on measurement of MTV in all target lesions, an approach that might be helpful in avoidance of an unnecessary overburden in the practice of nuclear medicine specialists.
Park et al. [15] reported a significant association of baseline SUVsum with survival in patients with diffuse large B-cell lymphoma (DLBCL). DLBCL is an aggressive tumor, which can arise in virtually any part of the body, like SCLC [16]. Similar to our result, metabolic burden represented by SUVsum from baseline 18F-FDG PET/CT was predictive of clinical outcome in patients with these aggressive tumors.
Results of our study showed that chemotherapy cycles and response to first-line treatment were good prognostic factors in SCLC. Because SCLC is extremely sensitive to chemotherapy, systemic chemotherapy is the main treatment strategy [14]. In this study, patients receiving chemotherapy more than three times had a significantly longer median OS and PFS than patients receiving chemotherapy once or twice (13.0 months vs. 4.3 months, p<0.001 and 7.7 months vs. 2.2 months, p<0.001, respectively). In addition, patients exhibiting CR or PR after the first treatment showed significantly longer median OS and PFS than patients exhibiting SD or PD (12.2 months and 9.7 months, p=0.001 and 7.0 months and 3.8 months, p<0.001, respectively). Historically, in many malignancies, including lung cancer, measurement of objective response has been an effective tool for prediction of OS [17,18,19,20]. Patients who did not enter CR or PR after two or three cycles of chemotherapy showed poor outcomes. Our findings may reflect the clinical importance of early switching of the chemotherapy regimen in patients who show poor response to first-line treatment. The results of a Japanese clinical trial indicated the benefit of an early switch from etoposide to irinotecan [21]. All of these findings indicate the need for development of new therapeutic strategies for patients without early tumor regression in order to improve their poor prognosis.
We recognize several limitations of this study. First, it was a retrospective review over a long period, which included a heterogeneous cohort and treatment protocols. However, this is unlikely to have had an impact on our results, because several studies have demonstrated thatthe prognosis of SCLC does not change significantly during and beyond the study period [2]. Second, in our study, tumor stage did not significantly predict survival. Only some of our patients with LD underwent concurrent thoracic radiation therapy, for various reasons. Early concurrent thoracic radiation therapy is known to improve OS and PFS in patients with LD [22]. This might explain our inability to find statistical significance regarding tumor stage in prediction of OS and PFS in our study population.
The current treatment strategy based on the stage of the disease is not completely satisfactory SCLC, and development of novel therapeutic approaches based on precise risk stratification is challenging. In this study, metabolic burden represented by SUVsum from baseline 18F-FDG PET/CT was an important independent prognostic factor in patients with SCLC. We suggest that SUVsum combined with tumor stage can more precisely predict clinical outcome in patients with SCLC.
Acknowledgments
This work was supported by a clinical research grant from the Biomedical Research Institute, Pusan National University Hospital (2013).
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. PMID: 21296855.

2. Aisner J. Extensive-disease small-cell lung cancer: the thrill of victory: the agony of defeat. J Clin Oncol. 1996; 14:658–665. PMID: 8636784.

3. Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997; 111:1710–1717. PMID: 9187198.

4. Yip D, Harper PG. Predictive and prognostic factors in small cell lung cancer: current status. Lung Cancer. 2000; 28:173–185. PMID: 10812187.

5. Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol. 1990; 8:1563–1574. PMID: 2167954.

6. Pillot G, Siegel BA, Govindan R. Prognostic value of fluorodeoxyglucose positron emission tomography in non-small cell lung cancer: a review. J Thorac Oncol. 2006; 1:152–159. PMID: 17409845.

7. Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008; 3:6–12. PMID: 18166834.

8. Ikenaga N, Otomo N, Toyofuku A, Ueda Y, Toyoda K, Hayashi T, et al. Standardized uptake values for breast carcinomas assessed by fluorodeoxyglucose-positron emission tomography correlate with prognostic factors. Am Surg. 2007; 73:1151–1157. PMID: 18092653.

9. Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004; 59:1295–1300. PMID: 15275712.

10. Lee YJ, Cho A, Cho BC, Yun M, Kim SK, Chang J, et al. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin Cancer Res. 2009; 15:2426–2432. PMID: 19318478.

11. Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011; 73:332–337. PMID: 21292341.

12. Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008; 49:480–508. PMID: 18287273.

13. Um SW, Kim H, Koh WJ, Suh GY, Chung MP, Kwon OJ, et al. Prognostic value of 18F-FDG uptake on positron emission tomography in patients with pathologic stage I non-small cell lung cancer. J Thorac Oncol. 2009; 4:1331–1336. PMID: 19701106.

14. Elias AD. Small cell lung cancer: state-of-the-art therapy in 1996. Chest. 1997; 112(4 Suppl):251S–258S. PMID: 9337299.
15. Park S, Moon SH, Park LC, Hwang DW, Ji JH, Maeng CH, et al. The impact of baseline and interim PET/CT parameters on clinical outcome in patients with diffuse large B cell lymphoma. Am J Hematol. 2012; 87:937–940. PMID: 22730093.

16. Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of disease. Philadelphia: Elsevier Saunders;2009.
17. Bruzzi P, Del Mastro L, Sormani MP, Bastholt L, Danova M, Focan C, et al. Objective response to chemotherapy as a potential surrogate end point of survival in metastatic breast cancer patients. J Clin Oncol. 2005; 23:5117–5125. PMID: 15955906.

18. Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Harita S, et al. Continued gefitinib treatment after disease stabilisation prolongs survival of Japanese patients with non-small-cell lung cancer: Okayama Lung Cancer Study Group experience. Ann Oncol. 2005; 16:1817–1823. PMID: 16157622.

19. Hotta K, Kiura K, Fujiwara Y, Takigawa N, Oze I, Ochi N, et al. Association between incremental gains in the objective response rate and survival improvement in phase III trials of first-line chemotherapy for extensive disease small-cell lung cancer. Ann Oncol. 2009; 20:829–834. PMID: 19221150.

20. Hotta K, Fujiwara Y, Matsuo K, Kiura K, Takigawa N, Tabata M, et al. Time to progression as a surrogate marker for overall survival in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009; 4:311–317. PMID: 19190515.

21. Kubota K, Nishiwaki Y, Sugiura T, Noda K, Mori K, Kawahara M, et al. Pilot study of concurrent etoposide and cisplatin plus accelerated hyperfractionated thoracic radiotherapy followed by irinotecan and cisplatin for limited-stage small cell lung cancer: Japan Clinical Oncology Group 9903. Clin Cancer Res. 2005; 11:5534–5538. PMID: 16061870.

22. Simon GR, Turrisi A. American College of Chest Physicians. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007; 132(3 Suppl):324S–339S. PMID: 17873178.
Fig. 1
Kaplan-Meier survival curves for overall survival (p<0.001) (A) and progression-free survival (p<0.001) (B) of the two groups, which were divided based on the median value of sum of standardized uptake value (SUVsum) of pretreatment 18F-fluorodeoxyglucose positron emission tomography/computed tomography of 114 patients with small cell lung cancer.
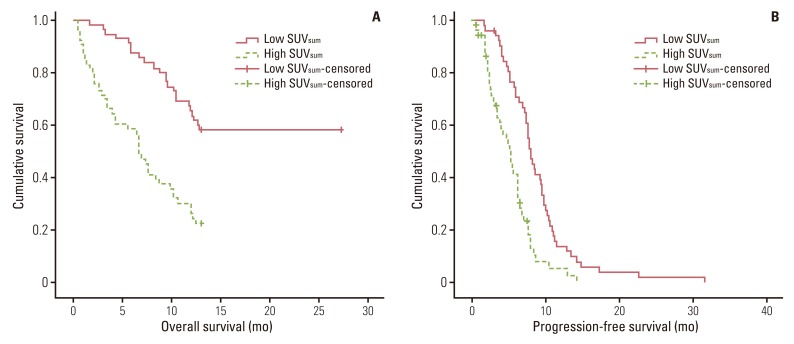
Fig. 2
Kaplan-Meier survival curves for progression-free survival (p<0.001) (A) and overall survival (p<0.001) (B) of four groups according to the combined criteria of sum of standardized uptake value (SUVsum) and tumor stage of 114 patients with small cell lung cancer.
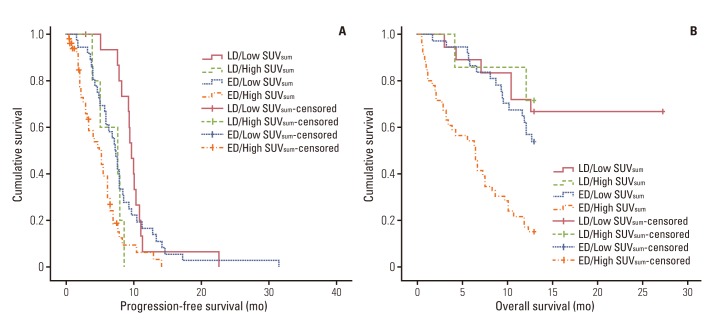
Table 1
Patient characteristics
| Variable | Total (n=114) | SUVsum<24.6a) (n=57) | SUVsum≥24.6a) (n=57) | p-value |
|---|---|---|---|---|
| Age (yr) | 0.248 | |||
| <65 | 44 (38.6) | 25 (43.9) | 19 (33.3) | |
| ≥65 | 70 (61.4) | 32 (56.1) | 38 (66.7) | |
| Gender, male | 105 (92.1) | 52 (91.2) | 53 (93.0) | 0.999 |
| Performance (ECOG) | 0.616 | |||
| 0, 1 | 95 (85.6) | 47 (83.9) | 48 (87.3) | |
| ≥2 | 16 (14.4) | 9 (16.1) | 7 (12.7) | |
| Tumor stage | 0.007 | |||
| LD | 26 (22.8) | 19 (33.3) | 7 (12.3) | |
| ED | 88 (77.2) | 38 (66.7) | 50 (87.7) | |
| Chemotherapy cycle | 0.138 | |||
| <3 | 17 (17.9) | 6 (12.2) | 11 (23.9) | |
| ≥3 | 78 (82.1) | 43 (87.8) | 35 (76.1) | |
| LDH (units/L) | 0.076 | |||
| <420 | 39 (34.2) | 24 (42.1) | 15 (26.3) | |
| ≥420 | 75 (65.8) | 33 (57.9) | 42 (73.7) | |
| NSE (ng/mL) | 0.010 | |||
| <28.5 | 31 (37.3) | 21 (51.2) | 10 (23.8) | |
| ≥28.5 | 52 (62.7) | 20 (48.8) | 32 (76.2) | |
| CYFRA21-1 (ng/mL) | 0.014 | |||
| <2.4 | 42 (44.7) | 26 (57.8) | 16 (32.7) | |
| ≥2.4 | 52 (55.3) | 19 (42.2) | 33 (67.3) |
Table 2
Multivariate analysis of survival
| Overall suvival | Progression-free survival | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (<65 yr vs. ≥65 yr) | 7.934 (2.579-24.413) | <0.001 | 0.968 (0.601-1.557) | 0.892 |
| Gender (female vs. male) | 2.796 (0.320-24.457) | 0.353 | 7.425 (2.119-26.010) | 0.002 |
| Stage (LD vs. ED) | 0.563 (0.190-1.664) | 0.299 | 1.105 (0.614-1.988) | 0.739 |
| Chemotherapy (≥3 vs. <3) | 17.701 (4.209-74.445) | <0.001 | 8.148 (3.482-19.066) | <0.001 |
| Response to first-line treatment (CR/PR vs. SD/PD) | 4.385 (1.329-14.463) | 0.015 | 1.894 (1.108-3.236) | 0.020 |
| LDH (<420 units/L vs. ≥420 units/L) | 1.583 (0.635-3.946) | 0.325 | - | - |
| NSE (<28.5 ng/mL vs. ≥28.5 ng/mL) | 2.511 (0.952-6.625) | 0.063 | - | - |
| CYFRA21-1 (<2.4 ng/mL vs. ≥2.4 ng/mL) | 1.020 (0.407-2.554) | 0.967 | - | - |
| SUVsum (<24.6a) vs. ≥24.6) | 3.970 (1.471-10.715) | 0.006 | 2.296 (1.388-3.797) | 0.001 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download