Abstract
Purpose
To evaluate the treatment outcomes of local excision following preoperative chemoradiotherapy in patients with locally advanced rectal cancer who have not undergone radical surgery for any reason.
Materials and Methods
The data of 27 patients with locally advanced rectal cancer who underwent preoperative chemoradiotherapy followed by local excision were analyzed retrospectively. The primary endpoint was the 5-year relapse-free survival rate, and the secondary endpoint was the pattern of recurrence.
Results
The median follow-up time was 81.8 months (range, 28.6 to 138.5 months). The 5-year local relapse-free survival (LRFS), distant metastasis-free survival (DMFS), relapse-free survival (RFS), and overall survival (OS) were 88.9%, 81.1%, 77.8%, and 85.0%, respectively. Six (22%) patients developed treatment failure; one (4%) patient had local recurrence only, three (11%) patients had distant recurrence only, and two (7%) patients had both. The 5-year LRFS, DMFS, RFS, and OS for patients with ypT0-1 compared with ypT2-3 were 94.1% vs. 77.8% (p=0.244), 94.1% vs. 55.6% (p=0.016), 88.2% vs. 55.6% (p=0.051), and 94.1% vs. 66.7% (p=0.073), respectively.
Local excision (LE) techniques for rectal cancer are appealing due to their lower perioperative morbidity and mortality rates and better functional outcomes compared with standard transabdominal resection. However, LE with curative intent is currently recommended for strictly selected T1 rectal cancers with favorable features such as <30% rectal circumference, <3 cm in size, mobile, non-fixed, within 8 cm of the anal verge, well to moderately differentiated, and no evidence of lymphadenopathy on pretreatment imaging [1]. Although several studies have reported the treatment results of LE for patients with cT2 tumors, LE has not gained widespread acceptance due to disappointing outcomes even with adjuvant therapy [2,3,4]. For more advanced tumors, LE should be restricted to palliative purposes only.
In the era of preoperative chemoradiotherapy (CRT), the potential advantages of preoperative CRT have increased interest in the use of LE for patients with locally advanced rectal cancer (LARC) who show significant tumor regression after preoperative CRT. Several retrospective studies have reported the treatment outcomes of LE for a highly select subset of patients with cT2-3 rectal cancer who respond well to preoperative CRT [5,6,7]. However, most reported data were limited by being assessed in only a few single-center studies involving relatively small numbers of patients with favorable features. A few prospective phase II trials of preoperative CRT and LE are ongoing. The American College of Surgeons Oncology Group (ACOSOG) trial Z6041 recently published preliminary results of preoperative CRT and LE for cT2N0 rectal cancer [8]. In addition, the Capecitabine, Radiotherapy and Transanal Endoscopic Microsurgery Surgery (CARTS) study, which will investigate the feasibility of preoperative CRT followed by transanal endoscopic microsurgery in patients with cT1-3N0 rectal cancer, is currently recruiting participants [9]. However, retrospective series or clinical trials regarding clinical experience with the use of preoperative CRT and LE for LARC are sparse.
This study investigated the treatment outcomes of LE following preoperative CRT for patients with cT3-4 rectal cancer who refused ablation of the anal sphincter, were considered inoperable due to medical comorbidities that contraindicated a major surgery, or achieved good response to preoperative CRT.
The patient database was reviewed to select patients with LARC who received preoperative CRT followed by LE. All patients were required to meet the following inclusion criteria for entry into the study: 1) histologically confirmed rectal adenocarcinoma, 2) cT3-4 classification, 3) no distant metastasis, and 4) the initiation of preoperative CRT before September 30, 2009. Eight Korean radiation oncology centers participated in the data collection, and a total of 27 patients were registered. There were mainly two situations in which LE was conducted. One was when the doctor recommended LE as the primary option limited to clinical complete response (CR) patients. The other was when the doctor recommended the procedure as an alternative to radical surgery due to refusal of radical surgery, old age, medical inoperability, etc. In the case of the former, the patient showing clinical CR following preoperative CRT would have given his or her consent to an experimental treatment after being sufficiently informed. This study was approved by the Institutional Review Board of the National Cancer Center, Republic of Korea (NCCNCS-12-649) and the Korean Radiation Oncology Group (KROG 12-04).
Preoperative radiation therapy was given to a dose of 44.0 to 50.4 Gy (median, 50.4 Gy) in 1.8 to 2.0 Gy daily fractions. Conventional or three-dimensional treatment planning using three- or four-field techniques was used. Chemotherapy was administered concurrently with radiation therapy using a fluoropyrimidine-based regimen (n=23, 85%) or irinotecan-based regimen (n=4, 15%). Four to 8 weeks after completion of preoperative CRT, LE was performed by a transanal approach under general anesthesia. Full-thickness excision of the tumor or scar with negative margins was performed. Nineteen patients (70%) received adjuvant chemotherapy comprising a fluoropyrimidine-based regimen (n=18, 67%) or oxaliplatin-based regimen (n=1, 4%). The chemotherapeutic regimens, both preoperative and postoperative, were selected for each patient according to the preferences of the attending medical oncologists.
All patients underwent a standard pretreatment workup including medical history, physical examination, serum carcinoembryonic antigen level, and imaging studies including chest X-ray and computed tomography (CT), transrectal ultrasonography, 18F-fluorodeoxyglucose positron emission tomography, or magnetic resonance imaging. The response to preoperative CRT was evaluated using the tumor regression grading system proposed by Dworak et al. [10]. Tumor regression was graded as follows: grade 0, no regression; grade 1, minimal regression; grade 2, moderate regression; grade 3, near-complete regression; and grade 4, complete regression. All tumors were staged according to the American Joint Committee on Cancer staging manual 7th edition.
All patients underwent standardized follow-up comprising a physical examination, digital rectal examination, complete blood counts, biochemical profiles, and serum carcinoembryonic antigen level at each visit. Follow-up imaging studies, such as abdominopelvic CT and colonoscopy, were performed as clinically indicated or at the physician's discretion. Disease recurrence was pathologically proven by surgical resection, biopsy, or cytology and/or radiological findings.
The primary endpoint was relapse-free survival (RFS), defined as the time interval from the initiation of CRT to any type of recurrence other than death. Local failure was defined as any disease recurrence within the pelvis, and any failure outside the pelvis was classified as a distant metastasis. Survival was estimated using the Kaplan-Meier method, with differences compared using the log-rank test. Hazard ratios were calculated using a Cox proportional-hazards model. Statistical significance was defined as a p-value of <0.05.
Table 1 shows the individual and clinical characteristics of the patients. At the time of diagnosis, all patients had an Eastern Cooperative Oncology Group performance status of 0 to 1. All tumors were assessed by digital rectal examination and were located within 7 cm of the anal verge. In the present study, the most common reasons for undergoing LE were good clinical response and doctor's recommendation (n=14, 52%) and patient's refusal of radical surgery (n=8, 30%), followed by old age (n=2, 7%), medical inoperability (n=2, 7%), and unknown (n=1, 4%). Two patients were considered medically inoperable owing to poor pulmonary function and cerebrovascular accident, respectively. The duration of CRT was 29 to 42 days (median, 37 days). One patient did not complete the planned radiation therapy (45 Gy) at a dose of 39.6 Gy due to severe enteritis. Another patient received three of six cycles of postoperative capecitabine due to a cardiovascular event. The pathologic tumor characteristics after LE are summarized in Table 2. ypT0 tumors were found in nine patients with negative resection margins.
The median follow-up time was 81.8 months (range, 28.6 to 138.5 months). The 5-year local relapse-free survival (LRFS), distant metastasis-free survival (DMFS), RFS, and overall survival (OS) were 88.9%, 81.1%, 77.8%, and 85.0%, respectively. Six (22%) patients developed treatment failure during the follow-up period: one (4%) patient had local recurrence only; three (11%) patients had distant recurrence only; and two (7%) patients had both. Among six patients with disease recurrence, three were alive at the last follow-up with a relapse-free status after salvage treatment: one patient (ypT0N0) who developed local recurrence only at the excision site after 14 months was salvaged with abdominoperineal resection (rpT2N0) and adjuvant chemotherapy (six cycles of 5-fluorouracil plus leucovorin); another patient with hepatic metastasis was salvaged with radiofrequency ablation and chemotherapy; and the other patient with both local and distant recurrences was treated with salvage chemotherapy (capecitabine plus oxaliplatin). During the study period until the time of analysis, a total of five (19%) patients died: three (11%) died of disease recurrence; one (4%) died of newly developed non-small-cell lung cancer; and one (4%) died of an unknown cause (no known recurrence). In this study, there were three patients who had resection margin positive. In one patient, recurrence did not occur during the follow-up period, and in the other two, it occurred only in a distant organ, thus our results indicated that margin positivity did not increase local recurrence.
Univariate analysis was performed to identify the prognostic value of all clinicopathologic factors listed in Tables 1 and 2. The 5-year OS (94.1% vs. 66.7%, p=0.073) (Fig. 1A) and RFS (88.2% vs. 55.6%, p=0.051) (Fig. 1B) were better in patients with ypT0-1 than in those with ypT2-3, respectively, with borderline significance. The 5-year LRFS rates for patients with ypT0-1 and ypT2-3 were 94.1% and 77.8%, respectively (p=0.244) (Fig. 2A). The DMFS rate of patients with ypT0-1 was significantly better than that of ypT2-3 (94.1% vs. 55.6%, respectively; p=0.016) (Fig. 2B). None of the other variables tested were significantly correlated with LRFS, DMFS, RFS, or OS.
The purpose of this study was to evaluate the treatment outcomes of patients with LARC who underwent LE following preoperative CRT. At present, radical surgery remains the mainstay of treatment for LARC. Nevertheless, there has been increasing interest in the use of LE as a fallback option for LARC as well as early rectal cancer. There are several reasons that LE can be performed in patients with LARC. Some patients who want a better functional outcome and quality of life may refuse radical surgery mostly due to nonacceptance of permanent colostomy. In addition, physicians may recommend LE for patients who are deemed medically inoperable.
However, one of the major problems related to LE for LARC is the higher recurrence rate. Two cooperative group trials have provided information on the local recurrence rates of LE followed by adjuvant CRT for LARC. The Radiation Therapy Oncology Group Protocol 89-02 evaluated the efficacy of LE in 38 patients with cT2-3 rectal cancer and reported that LE was a feasible alternative treatment but that the local recurrence rates (16% in T2 and 23% in T3) and distant dissemination rates (12% in T2 and 31% in T3) appeared to escalate with increasing T classification [2]. The Cancer and Leukemia Group B (CALGB) 8,984 study reported the updated outcomes of 51 patients with T2 rectal cancer. The 10-year OS rate and local recurrence rate were 64% and 18%, respectively [3]. Thus, the high rate of local recurrence with LE remains unresolved, and the advantages of LE should be balanced against this high local recurrence rate.
Preoperative CRT is well known to be effective in reducing the local recurrence rate [11]. Several studies have shown promising results for LE after preoperative CRT in patients with select cT2-3 tumors that responded well to CRT. Preoperative CRT allows downstaging of the primary tumor, sterilization of micrometastases in the pelvis, improved locoregional control, and decreased complication rates. Among these, significant tumor responses to preoperative CRT may lead to the use of LE even in patients at a high risk of disease recurrence because a pathologically complete response (ypCR) after CRT was suggested to have a favorable outcome [12,13,14]. The key rationale for this approach is the correlation between radiosensitivity and the inherent low aggressiveness of rectal cancer [15]. Bonnen et al. [16] demonstrated that local control and survival rates of patients treated with CRT followed by LE were comparable to those of patients treated with CRT followed by mesorectal excision. Borschitz et al. [17] analyzed previously published data regarding LE after preoperative CRT for cT2-3 rectal cancer. The local recurrence rate of patients with ypT1 disease consistently showed a low incidence of 2% (range, 0 to 6%), whereas those in patients with ypT2 and ypT3 were 6% to 20% and up to 42%, respectively [17]. Mohiuddin et al. [18] reported that selected patients with cT3 tumors that were downstaged and met the criteria for LE appeared to have excellent survival outcomes. Our study also showed that patients with ypT0-1 tumors had a trend toward better treatment outcomes than those with ypT2-3 tumors. The 5-year local recurrence and OS rates in the present study for patients with ypT0-1 tumors were 5.9% and 94.1%, respectively, which were comparable to those reported for patients who underwent total mesorectal excision for early rectal cancer [19,20].
We included 12 (44%) patients with clinically positive pelvic lymph node(s) who were diagnosed by one or more imaging studies. If lymph node metastasis cannot be sterilized with preoperative CRT, LE in these patients may result in increased risks of local and distant recurrence because the residual lymphatic metastasis would have been left unresected. However, it is difficult to evaluate the status of lymph node involvement after preoperative CRT because LE did not provide information on ypN classification, and conventional imaging studies have limitations in assessing the response to preoperative CRT for rectal cancer due to radiation-induced edema, inflammation, and fibrosis [21]. Kim et al. [22] reported that pathologic nodal classification is an important prognostic factor for survival outcomes in patients with rectal cancer treated with preoperative CRT, and the ypT classification has been suggested as the most reliable predictor of ypN status: the ypN+ rate was 3.4% in ypT0-1; 16.9% in ypT2; and 49.3% in ypT3 patients [23]. Although the ypN classification was not reported in the present study, ypT classification may help to predict ypN status.
We have confirmed an association between the tumor responses to preoperative CRT and treatment outcomes. The results of the present study suggest that LE may be a reasonable alternative treatment for patients with LARC who achieve ypT0-1 after preoperative CRT. Although the treatment outcome of LE in this patient subset seems promising, careful consideration should be given to preoperative patient selection. We hope that the results described here will add to the growing evidence and guide any future changes in the use of LE for LARC.
Acknowledgments
This work was supported by a National Cancer Center Grant (NCC-1310070). The authors thank Dr. Min Kyu Kang at Yeungnam University, Dr. Woong Sub Koom at Yonsei University, Dr. Eui Kyu Chie at Seoul National University, and Dr. Jin Hee Kim at Keimyung University for patient accrual.
References
1. NCCN Clinical practice guidelines in oncology: rectal cancer [Internet]. Fort Washington: National Comprehensive Cancer Network;cited 2013 Mar 1. Available from: http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
2. Russell AH, Harris J, Rosenberg PJ, Sause WT, Fisher BJ, Hoffman JP, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of radiation therapy oncology group protocol 89-02. Int J Radiat Oncol Biol Phys. 2000; 46:313–322. PMID: 10661337.

3. Greenberg JA, Shibata D, Herndon JE 2nd, Steele GD Jr, Mayer R, Bleday R. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum. 2008; 51:1185–1191. PMID: 18536973.

4. Chakravarti A, Compton CC, Shellito PC, Wood WC, Landry J, Machuta SR, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999; 230:49–54. PMID: 10400036.

5. Nair RM, Siegel EM, Chen DT, Fulp WJ, Yeatman TJ, Malafa MP, et al. Long-term results of transanal excision after neoadjuvant chemoradiation for T2 and T3 adenocarcinomas of the rectum. J Gastrointest Surg. 2008; 12:1797–1805. PMID: 18709419.

6. Kim CJ, Yeatman TJ, Coppola D, Trotti A, Williams B, Barthel JS, et al. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg. 2001; 234:352–358. PMID: 11524588.

7. Yeo SG, Kim DY, Kim TH, Kim SY, Chang HJ, Park JW, et al. Local excision following pre-operative chemoradiotherapy-induced downstaging for selected cT3 distal rectal cancer. Jpn J Clin Oncol. 2010; 40:754–760. PMID: 20457724.

8. Garcia-Aguilar J, Shi Q, Thomas CR Jr, Chan E, Cataldo P, Marcet J, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012; 19:384–391. PMID: 21755378.

9. Bokkerink GM, de Graaf EJ, Punt CJ, Nagtegaal ID, Rutten H, Nuyttens JJ, et al. The CARTS study: chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery. BMC Surg. 2011; 11:34. PMID: 22171697.

10. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997; 12:19–23. PMID: 9112145.

11. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012; 30:1926–1933. PMID: 22529255.

12. Yeo SG, Kim DY. An update on preoperative radiotherapy for locally advanced rectal cancer. J Korean Soc Coloproctol. 2012; 28:179–187. PMID: 22993703.

13. Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg. 2010; 252:998–1004. PMID: 21107110.
14. Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008; 72:99–107. PMID: 18407433.

15. Bujko K, Sopylo R, Kepka L. Local excision after radio (chemo) therapy for rectal cancer: is it safe? Clin Oncol (R Coll Radiol). 2007; 19:693–700. PMID: 17766096.
16. Bonnen M, Crane C, Vauthey JN, Skibber J, Delclos ME, Rodriguez-Bigas M, et al. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys. 2004; 60:1098–1105. PMID: 15519780.

17. Borschitz T, Wachtlin D, Mohler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008; 15:712–720. PMID: 18163173.

18. Mohiuddin M, Marks G, Bannon J. High-dose preoperative radiation and full thickness local excision: a new option for selected T3 distal rectal cancers. Int J Radiat Oncol Biol Phys. 1994; 30:845–849. PMID: 7960986.

19. Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007; 246:693–701. PMID: 17968156.
20. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001; 345:638–646. PMID: 11547717.

21. Zeestraten EC, Kuppen PJ, van de Velde CJ, Marijnen CA. Prediction in rectal cancer. Semin Radiat Oncol. 2012; 22:175–183. PMID: 22385923.

22. Kim TH, Chang HJ, Kim DY, Jung KH, Hong YS, Kim SY, et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys. 2010; 77:1158–1165. PMID: 19800178.

23. Kim DW, Kim DY, Kim TH, Jung KH, Chang HJ, Sohn DK, et al. Is T classification still correlated with lymph node status after preoperative chemoradiotherapy for rectal cancer? Cancer. 2006; 106:1694–1700. PMID: 16532432.

Fig. 1
Comparison of overall survival curves (p=0.073) (A) and relapse-free survival curves (p=0.051) (B) in patients stratified by ypT status (solid line, ypT0-1; dashed line, ypT2-3).
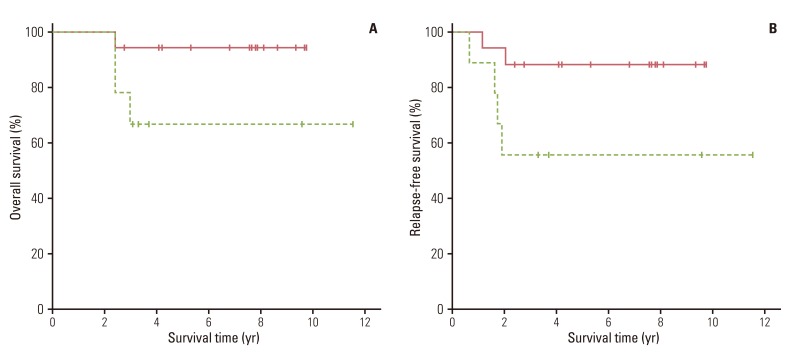
Fig. 2
Comparison of local recurrence-free survival curves (p=0.244) (A) and distant metastasis-free survival curves (p=0.016) (B) in patients stratified by ypT status (solid line, ypT0-1; dashed line, ypT2-3).

Table 1
Baseline patient and tumor characteristics
Table 2
Pathologic tumor characteristics




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download