Abstract
Purpose
This study was designed to evaluate the impact of radiochemotherapeutic sequence and time to initiation of adjuvant treatment on loco-regional control for resected stage II and III rectal cancer.
Materials and Methods
Treatment outcomes for rectal cancer patients from two hospitals with different sequencing strategies regarding adjuvant concurrent radiochemotherapy (CRCT) were compared retrospectively. Pelvic radiotherapy was administered concurrently on the first (early CRCT, n=180) or the third cycle of chemotherapy (late CRCT, n=180). During radiotherapy, two cycles of fluorouracil were provided to patients in both groups. In the early CRCT group, median six cycles of fluorouracil and leucovorin were prescribed during the post-CRCT period. In the late CRCT group, two cycles of fluorouracil were administered in the pre- and post-CRCT periods.
Results
No significant differences in the 5-year loco-regional recurrence-free survival (LRRFS) (92.5% vs. 95.6%, p=0.43) or overall survival and disease-free survival were observed between groups. Patients who began receiving adjuvant treatment later than five weeks after surgery had lower LRRFS than patients who received adjuvant treatment within five weeks following surgery (79% vs. 91%, p<0.01). The risk of loco-regional recurrence increased as the time to initiation of adjuvant treatment was delayed.
Conclusion
In the current study, treatment outcomes were not significantly influenced by the sequence of adjuvant treatment but by the delay of adjuvant treatment for more than five weeks. Timely administration of adjuvant treatment is deemed important in achieving loco-regional tumor control for stage II/III rectal cancer patients.
Surgical resection is a primary treatment for rectal cancer. However, almost 30% of patients with locally advanced rectal cancer develop pelvic recurrence after surgery alone [1]. Through the addition of adjuvant concurrent radiochemotherapy (CRCT) after surgery, loco-regional recurrence (LRR) rate was lowered by approximately 50% compared to patients who underwent surgery alone [2,3]. Based on this result, postoperative CRCT following surgery has been regarded as a standard treatment for locally advanced rectal cancer. More recently, there has been a tendency of using preoperative application of CRCT in an attempt to preserve the anal sphincter [4,5]. Nonetheless, some situations can be managed more prudently with initial surgery. When initial surgery is performed and adjuvant treatment is required, optimizing the combination of radiotherapy and chemotherapy is necessary to enhance effects of adjuvant treatment.
Deciding on a sequence of CRCT and a time to initiation of adjuvant treatment after surgery can be a practical problem in cases of rectal cancer treated with adjuvant CRCT. Most trials of post-operative CRCT for resected rectal cancer have used a sandwich technique in which one or two cycles of chemotherapy are followed by CRCT and then additional chemotherapy [3,6]. However, the optimal sequence of radiotherapy and chemotherapy is still unknown. In addition, permissible delay in initiation of adjuvant treatment has yet to be elucidated.
Two cancer centers in Korea have traditionally applied different sequencings of postoperative CRCT for locally advanced rectal cancer. The treatment sequence at Seoul National University Hospital has been immediate CRCT followed by adjuvant chemotherapy, while Samsung Medical Center has adopted administration of two cycles of chemotherapy followed by CRCT and two additional cycles of chemotherapy. We conducted a retrospective review of patients from the two hospitals to evaluate the impact of radiochemotherapeutic sequence and time to initiation of adjuvant treatment on loco-regional control for resected stage II and III rectal cancer.
The number of patients who underwent postoperative CRCT for rectal adenocarcinoma from August 1999 to March 2007 at Seoul National University Hospital (SNUH) and at Samsung Medical Center (SMC) was 334 and 664, respectively. For the retrospective cohort-matched comparison of the different sequences of postoperative radiotherapy and chemotherapy, 180 patients from each hospital were selected based on the pathologic stage. The inclusion criteria were as follows: 1) R0 resection with neither gross nor microscopic residual disease on the pathologic examination; 2) non-peritonealized tumors either located within 12 cm from the anal verge on pre-treatment evaluation or verified intra-operatively; 3) tumor extension through the bowel wall (pT3/T4) or regional lymph node involvement (pN1-2), but without distant metastasis (M0); 4) completion of pelvic radiotherapyper plan; 5) 5-fluorouracil (5-FU) or 5-FU and leucovorin (LV) for chemotherapeutic agent; 6) no previous medical history of other malignancies; 7) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 before starting adjuvant therapy; 8) normal hepatic, renal, and bone marrow function defined as bilirubin level<2.0 mg/dL, creatinine level<1.5 mg/dL, leukocyte count>4,000/µL, and platelet count>130,000/µL; and 9) post-treatment follow-up period of more than six months.
All patients underwent total mesorectal excision followed by adjuvant CRCT. Adjuvant treatment was administered using different sequences at each hospital. At SNUH, pelvic radiotherapy was administered concurrently with the first chemotherapy cycle following surgery (early CRCT group). Chemotherapy regimen during CRCT consisted of intravenous bolus 5-FU (500 mg/m2/day) for three days during weeks 1 and 5 of pelvic radiotherapy. After CRCT, median six cycles (range, 6 to 10 cycles) of bolus injection of 5-FU (375 mg/m2/day) and LV (20 mg/m2/day) for five days were added. At SMC, pelvic radiotherapy was administered during the third chemotherapy cycle (late CRCT group). Before CRCT, two cycles of bolus 5-FU (500 mg/m2/day) were administered for five days. The dose of chemotherapy during CRCT was the same as that used at SNUH. After CRCT, two more cycles of bolus 5-FU (500 mg/m2/day) were administered for five days (Fig. 1).
Target delineation and the portal arrangement of pelvic radiotherapy in two hospitals were quite homogenous. The primary tumor bed with the surrounding soft tissues, and the regional lymphatic chains were irradiated using a three field technique (posterior and two lateral fields). All patients received 45 Gy in 25 fractions over five weeks. Boost radiation to the tumor bed was added in all patients in the early CRCT group and 57 patients (31.6%) in the late CRCT group. The median radiation doses of the early and late CRCT group were 50.4 Gy (range, 45 to 56.4 Gy), and 45 Gy (range, 45 to 51 Gy), respectively.
History taking, physical examination, including digital rectal examination, complete blood count, carcinoembryonic antigen, blood chemistry, and chest X-ray were performed every 3-4 months for the first two years, and repeated every six months for the third year, and annually thereafter. Computed tomography of the abdomen and pelvis was performed every six months for the first two years, and then annually thereafter. Colonoscopic examination was repeated biennially. The median follow-up intervals were 62 months (range, 10 to 117 months) in the early CRCT group and 72 months (range, 7 to 116 months) in the late CRCT group. The grade of treatment related toxicity was determined by the National Cancer Institute Common Toxicity Criteria, ver. 2.0 [7].
Overall survival (OS), disease-free survival (DFS), and loco-regional recurrence-free survival (LRRFS) were defined as the intervals from surgery to death, disease relapse, and LRR, respectively. The LRR was defined as any recurrence within the pelvic radiation field excluding the peritoneal seeding. Chi-square test was used to compare the patient characteristics between two groups. The survival data were computed using the Kaplan-Meier method, and the log-rank test was used to compare the survival outcomes between groups with different variables. Cox regression analysis with stepwise selection was used to determine the independent prognostic factors for the outcomes. Statistical significance was calculated at the 95% confidence interval (p<0.05), and all analyses were performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL).
Data on tumors and patient characteristics are shown in Table 1. We categorized the involved lymph node ratio based on the frequency distribution to 0, 0-0.3, and >0.3. No significant difference with respect to age, gender, tumor location, types of resection, histologic grade, pathologic stage, and the ratio of involved lymph nodes was observed between the two groups. However, the time interval between surgery and initiation of adjuvant treatment, and the distal resection margin of tumor were different between the groups. More patients received adjuvant treatment within five weeks after surgery in the late CRCT group than in the early CRCT group (93% vs. 76%, p=0.01); and more patients in the early CRCT group had tumors with a close distal margin (<1 cm) than patients in the late CRCT group (24% vs. 6%, p=0.01).
The 5-year OS, DFS, and LRRFS rates were 81.0%, 67.1%, and 89.7% for all patients, respectively. Recurrence was found in 116 patients (32.2%). LRR as the first site of recurrence occurred in eight and four patients in the early and the late CRCT groups, and distant metastasis was found in 37 and 47 patients in the early and late CRCT groups, respectively. Simultaneous occurrence of LRR and distant metastasis was found in 15 and seven patients in the early and late CRCT groups, respectively. Data from analysis of the prognostic effects of variable factors on LRRFS are shown in Table 2. In univariate analysis, poor prognostic factors for LRRFS included distal rectal tumor, close distal resection margin defined as less than 1 cm, advanced pathologic stage, longer delay in initiation of adjuvant treatment defined as more than five weeks, and early CRCT. In multivariate analysis, close distal resection margin, advanced pathologic stage, and longer delay showed a significant association with lower 5-year LRRFS rate.
In univariate analysis, patients treated with early CRCT showed a significantly lower 5-year LRRFS rate than patients with late CRCT (85% vs. 94%, p=0.01). However, aforementioned difference in LRRFS between the early and the late CRCT groups was due to imbalances of characteristics between the two groups. As shown in Table 1, the proportions of patients with close distal resection margin and patients with delayed adjuvant treatment of more than five weeks after surgery were significantly higher in the early CRCT group than in the late CRCT group. Close distal resection margin and longer time to initiation of adjuvant treatment following surgery were important negative prognostic factors for LRRFS. Therefore, we re-analyzed the effect of radiochemotherapeutic sequence on the outcome according to the status of distal resection margin and the time to initiation of adjuvant treatment. LRRFS did not differ significantly between the early and late CRCT groups in accordance with distal margin status of tumor or the time to adjuvant treatment (Fig. 2).
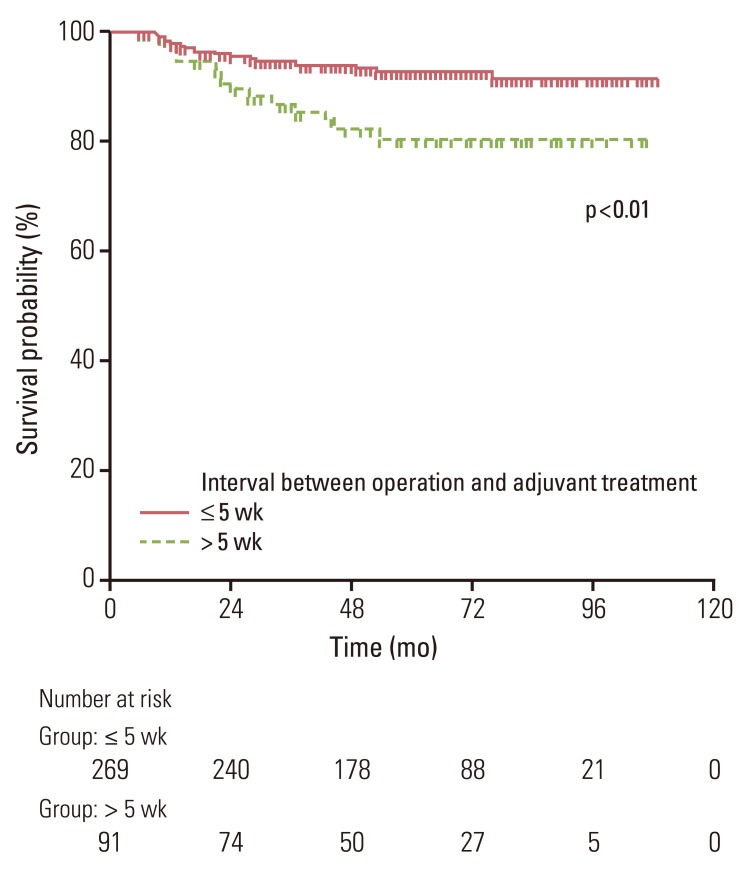
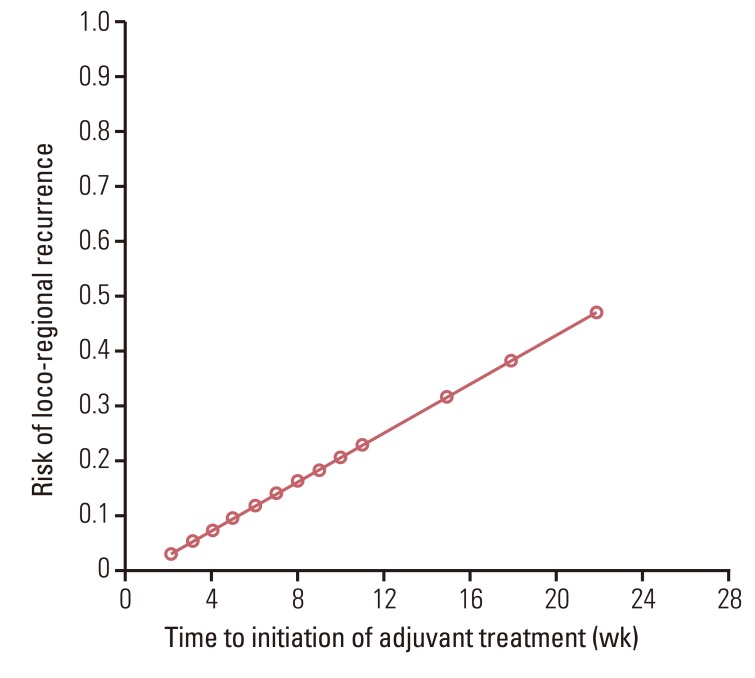
Delayed initiation of adjuvant treatment for more than five weeks was a significant adverse prognostic factor for LRRFS (79% vs. 91%, p<0.01) (Table 2, Fig. 3). Comparison of characteristics between the groups based on the time to initiation of adjuvant treatment is shown in Table 3. More patients in the early CRCT group had delay of adjuvant treatment of more than five weeks than patients in the late CRCT group. The risk of LRR was increased as the time to initiation of adjuvant treatment was delayed (Fig. 4). Neither OS nor DFS was affected by radiochemotherapeutic sequence or delay of adjuvant treatment. Pathologic stage and type of operation were significant factors for OS and DFS (Table 4).
Treatment related complications in relation to sequence of CRCT are shown in Table 5. Two patients in the early CRCT group had surgical complications before initiation of adjuvant treatment. One patient had grade 3 wound complication and the other had grade 2 ileus. Adjuvant treatment was initiated 59 days and 28 days after the operation for postoperative recovery, respectively.
Findings of the current study show that the timing of adjuvant treatment but not the radiochemotherapeutic sequence significantly affects loco-regional control of patients with locally advanced rectal cancer. An increase in LRR was observed among patients with delayed initiation of adjuvant treatment. However, the treatment outcome was not affected by sequence of adjuvant radiotherapy and chemotherapy. Since the report by the North Central Cancer Treatment Group (NCCTG), application of two cycles of chemotherapy followed by CRCT and additional chemotherapy has been regarded as a standard sequence of adjuvant treatment for resected rectal cancer [3]. This treatment scheme was aimed at administering full dose chemotherapy during the early postoperative period for effective eradication of probable distant metastasis. However, in practice, there are clinicians who administer pelvic radiotherapy with the first chemotherapy cycle to achieve early eradication of residual tumor cells within the pelvic cavity. For example, according to the Korean patterns of care study, one third of Korean rectal cancer patients received immediate postoperative CRCT followed by adjuvant chemotherapy [8]. Defining optimal sequence of radiotherapy and chemotherapy is a prerequisite for maximizing the therapeutic effect of adjuvant treatment. However, only one trial evaluating treatment outcomes in relation to adoption of different sequences of adjuvant treatment in resected rectal cancer patients has been reported.
In the study reported by Lee et al. [9], 308 patients with resected stage II/III rectal cancer were randomly assigned to receive pelvic radiation on either the first or the third course of LV-modulated 5-FU chemotherapy [10]. In the preliminary report, significantly higher DFS rate was achieved in the early pelvic radiotherapy group (81% vs. 70% at four years, p=0.047) [9]. However, with extended follow-up time, no significant difference in terms of DFS (71% vs. 63% at 10 years, p=0.162), OS (66% vs. 64% at 10 years, p=0.652), and relapse rate (27% vs. 35%, at 10 years) was observed between the two groups. As in the report by Lee et al. [9], current study failed to define the role of treatment sequencing. In the present study, margin status of tumor and waiting time for adjuvant treatment were not equally distributed between the early and late CRCT groups. As the aforementioned factors were important prognosticators for loco-regional control, we compared the outcome between the early and the CRCT groups with respect to distal resection margin of tumor and time to adjuvant treatment. Radiochemotherapeutic sequence did not affect loco-regional tumor control irrespective of given margin length or time to adjuvant treatment. Physicians tend to prefer immediate pelvic irradiation after surgery for patients with a close resection margin, with the aim of early elimination of residual tumor cells within the pelvic cavity. Nonetheless, present study found that loco-regional tumor control rate did not differ significantly according to the sequence of adjuvant treatment even in patients with a close distal tumor resection margin.
Of note, more patients in the early radiotherapy group waited more than five weeks before adjuvant treatment compared with the late radiotherapy group. The median time to adjuvant treatment was one week earlier in the late CRCT group than in the early CRCT group. In both the early and late CRCT groups, adjuvant treatments were planned to initiate within six weeks after surgery. However, in real clinical situations, the planned intervals between operation and adjuvant therapy were prone to be longer than the scheduled time. The reason why adjuvant treatment was delayed more frequently in the early CRCT group was uncertain. Medical records on the aforesaid subject were unavailable in most patients, except for the two who needed time for recovery from postoperative complication before initiation of adjuvant treatment. Delayed initiation of adjuvant therapy in the early CRCT group might be attributed to physician and patient factors. Presumably, physicians were likely to wait a longer period of time for postoperative recovery before initiation of pelvic radiotherapy in comparison to the situation involving administration of upfront chemotherapy after surgery. In addition, patients' preference or individual compliance with physician's advice could have affected time to adjuvant treatment. Perhaps, this one-week difference in adjuvant treatment initiation may merely be a reflection of time required for radiotherapy planning. Unlike chemotherapy, where treatment could be readily initiated, radiotherapy requires time for CT-based simulation followed by target delineation and radiation treatment planning, which takes approximately three days to one week in routine clinical practice. Due to their comparable outcomes, both sequences of early or late CRCT can be adopted as adjuvant therapy for rectal cancer. However, according to the results of the current study, delayed initiation of adjuvant treatment for more than five weeks showed an association with unsatisfactory loco-regional tumor control; therefore, selection of the sequence of adjuvant therapy should be based on the clinical situation with intent to timely initiation of adjuvant treatment.
A few studies have assessed the prognostic impact of time to adjuvant treatment in colorectal cancer [11,12,13,14,15]. In such studies, delayed initiation of adjuvant therapy of over certain durations was deemed to be related to lower survival rate. Most of such studies focused on colon or rectal cancer patients. The only study whose focus was confined to rectal cancer patients reported that initiating adjuvant therapy more than three months after surgery resulted in worse DFS and OS than initiating the therapy within three months following the operation [12]. Present study also found that time to adjuvant treatment significantly affected LRRFS, although no relation was established between time to adjuvant treatment and DFS or OS. A high LRR rate of 21% in five years was observed for patients who started adjuvant treatment more than five weeks after surgery. This rate of LRR is relatively higher compared with other reports using similar 5-FU based CRCT with timely administration of adjuvant treatment [6,10]. Delay in adjuvant therapy could provide a more favorable environment for growth of residual tumor foci. In addition, delays in adjuvant treatment raised the probability of mutations that might lead to drug resistance [15,16]. Consequently, prolonged time to adjuvant treatment is negatively associated with loco-regional tumor control. The cutoff delay in the current analysis was shorter than that of other studies reporting a cutoff point of four to 16 weeks [14,15]. This was because most patients in the current analysis received adjuvant treatment within eight weeks after surgery. Therefore, the cutoff time for discrimination between groups with favorable and unfavorable outcomes might be earlier than that of previous studies.
Our research has limitations inherent to retrospective comparison of clinical outcomes between two facilities. Differences in surgical quality and non-identical chemotherapeutic agents between the two hospitals could have affected loco-regional tumor control. Distal resection margin length of tumor was shown to be important in the current study, however, radial resection margin, which is also reported to be influential was not taken into account [17,18,19]. Likewise, current analysis on the effect of CRCT sequence on treatment outcome could have been biased by such abovementioned differences between the two facilities. Further prospective randomized study is necessary for accurate evaluation of the prognostic impact of radiochemotherapeutic sequence on treatment outcome for patients treated with adjuvant CRCT. Nonetheless, conduct of such a randomized controlled trial is quite unlikely because a larger number of patients with locally advanced rectal cancer have been treated with preoperative CRCT rather than postoperative CRCT since publication of the German trial [4,5].
Our research demonstrated that treatment outcome was not significantly influenced by the sequence of adjuvant treatment in stage II and III rectal cancer. Either early or late administration of pelvic radiotherapy resulted in comparable tumor control. A significant adverse association was observed between time to adjuvant therapy and loco-regional tumor control. Patients with delayed initiation of postoperative adjuvant treatment of more than five weeks showed unfavorable loco-regional control. Timely administration of adjuvant treatment is important to achieve loco-regional tumor control for rectal cancer patients.
References
1. McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis. 1995; 10:126–132. PMID: 7561427.

2. Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985; 312:1465–1472. PMID: 2859523.
3. Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991; 324:709–715. PMID: 1997835.

4. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740. PMID: 15496622.

5. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012; 30:1926–1933. PMID: 22529255.

6. Smalley SR, Benedetti JK, Williamson SK, Robertson JM, Estes NC, Maher T, et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol. 2006; 24:3542–3547. PMID: 16877719.

7. Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000; 47:13–47. PMID: 10758303.

8. Kim JH, Oh DH, Kang KM, Kim WC, Kim WD, Kim JS, et al. Postoperative radiotherapy in the rectal cancers patterns of care study for the years of 1998~1999. J Korean Soc Ther Radiol Oncol. 2005; 23:22–31.
9. Lee JH, Lee JH, Ahn JH, Bahng H, Kim TW, Kang YK, et al. Randomized trial of postoperative adjuvant therapy in stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: a preliminary report. J Clin Oncol. 2002; 20:1751–1758. PMID: 11919231.

10. Kim TW, Lee JH, Lee JH, Ahn JH, Kang YK, Lee KH, et al. Randomized trial of postoperative adjuvant therapy in Stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: 10-year follow-up. Int J Radiat Oncol Biol Phys. 2011; 81:1025–1031. PMID: 20932669.

11. Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005; 16:549–557. PMID: 15695501.

12. Cheung WY, Neville BA, Earle CC. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Dis Colon Rectum. 2009; 52:1054–1063. PMID: 19581846.

13. Ahmed S, Ahmad I, Zhu T, Arnold FP, Faiz Anan G, Sami A, et al. Early discontinuation but not the timing of adjuvant therapy affects survival of patients with high-risk colorectal cancer: a population-based study. Dis Colon Rectum. 2010; 53:1432–1438. PMID: 20847626.

14. Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010; 46:1049–1055. PMID: 20138505.

15. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011; 305:2335–2342. PMID: 21642686.
16. Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979; 63:1727–1733. PMID: 526911.
17. Dent OF, Haboubi N, Chapuis PH, Chan C, Lin BP, Wong SK, et al. Assessing the evidence for an association between circumferential tumour clearance and local recurrence after resection of rectal cancer. Colorectal Dis. 2007; 9:112–121. PMID: 17223934.

18. Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994; 344:707–711. PMID: 7915774.

19. Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH. Pathology Review Committee. Cooperative Clinical Investigators. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002; 26:350–357. PMID: 11859207.
Fig. 1
Treatment scheme. CRCT, concurrent radiochemotherapy; 5-FU, 5-fluorouracil; RT, radiotherapy.
Fig. 2
Loco-regional recurrence-free survival (LRRFS) between the early and late concurrent radiochemotherapy (CRCT) groups according to resection margin (RM) and time to initiation of adjuvant treatment (TTA).
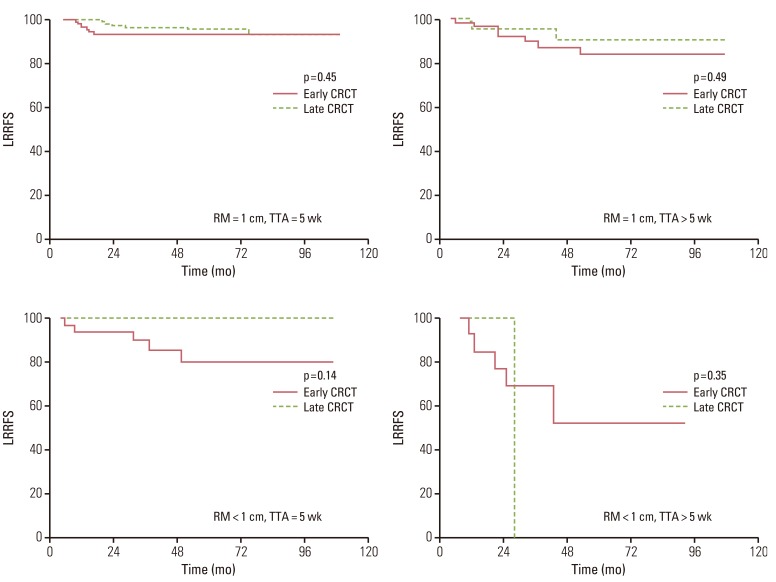
Table 1
Patients and tumor characteristics between the early and late CRCT groups
| Characteristic | Early CRCT | Late CRCT | p-value |
|---|---|---|---|
| Age (yr) | |||
| ≤55 | 78 (43) | 74 (41) | 0.74 |
| >55 | 102 (57) | 106 (59) | |
| Gender | |||
| Male | 127 (70) | 114 (63) | 0.17 |
| Female | 53 (30) | 66 (37) | |
| Tumor location | |||
| ≤5 cm from anal verge | 55 (30) | 58 (32) | 0.82 |
| >5 cm from anal verge | 125 (70) | 122 (68) | |
| Distal resection margin (cm) | |||
| <1 | 44 (24) | 12 (6) | 0.01 |
| ≥1 | 136 (76) | 170 (94) | |
| Differentiation | |||
| Well differentiated | 24 (13) | 15 (8) | 0.27 |
| Moderately differentiated | 144 (80) | 147 (81) | |
| Poorly differentiated | 3 (1) | 7 (3) | |
| Unknown | 9 (6) | 11 (8) | |
| Pathologic stage | |||
| II | 67 (37) | 67 (37) | Matched |
| IIIA | 8 (5) | 8 (5) | |
| IIIB | 53 (29) | 53 (29) | |
| IIIC | 52 (29) | 52 (29) | |
| Ratio of involved lymph nodesa) | |||
| 0 | 67 (37) | 67 (37) | 0.92 |
| >0 and ≤0.3 | 57 (32) | 60 (33) | |
| >0.3 | 56 (31) | 53 (30) | |
| Types of resection | |||
| LAR | 157 (87) | 143 (79) | 0.06 |
| APR | 23 (13) | 37 (21) | |
| Time to adjuvant treatment Median (range) weeks | 5 (3-22) | 4 (2-15) | |
| ≤5 | 113 (62) | 156 (86) | 0.01 |
| >5 and ≤8 | 58 (33) | 20 (12) | |
| >8 | 9 (5) | 4 (2) |
Table 2
Univariate and multivariate analysis of prognostic factors for LRRFS
Table 3
Patients and tumor characteristics according to time to initiation of AT
| Characteristic | Time to AT≤5 wk | Time to AT>5 wk | p-value |
|---|---|---|---|
| Age (yr) | |||
| ≤55 | 114 (42) | 38 (42) | 0.98 |
| >55 | 155 (58) | 53 (58) | |
| Gender | |||
| Male | 174 (64) | 57 (62) | 0.82 |
| Female | 95 (36) | 34 (38) | |
| Distance from anal verge (cm) | |||
| ≤5 | 80 (29) | 33 (36) | 0.31 |
| >5 | 188 (71) | 58 (64) | |
| Types of resection | |||
| LAR | 225 (83) | 76 (83) | 0.89 |
| APR | 44 (17) | 15 (17) | |
| Differentiation | |||
| Well differentiated | 31 (11) | 8 (8) | 0.57 |
| Moderately differentiated | 219 (81) | 72 (79) | |
| Poorly differentiated | 6 (4) | 1 (7) | |
| Unknown | 12 (4) | 6 (6) | |
| Pathologic stage | |||
| II | 104 (38) | 30 (32) | 0.39 |
| III | 165 (62) | 61 (68) | |
| Ratio of involved lymph nodesa) | |||
| 0 | 104 (38) | 30 (32) | 0.48 |
| >0 and ≤0.3 | 105 (39) | 40 (44) | |
| >0.3 | 60 (23) | 21 (24) | |
| Distal resection margin (cm) | |||
| <1 | 41 (15) | 15 (16) | 0.68 |
| ≥1 | 228 (85) | 76 (84) | |
| Sequence of adjuvant treatment | |||
| Early CRCT | 113 (42) | 67 (73) | <0.01 |
| Late CRCT | 156 (58) | 24 (27) |
Table 4
Significant prognostic factors for OS and DFS by multivariate analysis
| Variable | OS | DFS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Types of operation | ||||||
| LAR vs. APR | 1.92 | 1.16-3.18 | 0.01 | 1.88 | 1.24-2.85 | <0.01 |
| Pathologic stage | ||||||
| II vs. III | 4.23 | 2.17-8.21 | <0.01 | 3.76 | 2.28-6.22 | <0.01 |
Table 5
Treatment-related complications according to different sequence of adjuvant treatment
| Characteristic | Early CRCT | Late CRCT | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Gr2 | Gr3 | Gr4 | Gr2 | Gr3 | Gr4 | |
| Before CRCTa) | ||||||
| Wound complication | 0 | 1 | 0 | 0 | 0 | 0 |
| Ileus | 1 | 0 | 0 | 0 | 0 | 0 |
| During/after CRCTb) | ||||||
| Nausea/vomiting | 0 | 0 | 0 | 9 | 0 | 0 |
| Diarrhea | 34 | 8 | 0 | 71 | 55 | 0 |
| Dermatitis | 6 | 2 | 0 | 11 | 0 | 0 |
| Neutropenia | 13 | 7 | 3 | 50 | 15 | 5 |
| Anemia | 6 | 1 | 0 | 9 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 5 | 1 | 0 |
| Wound complication | 0 | 2 | 0 | 0 | 0 | 0 |
| Ileus | 6 | 8 | 3 | 0 | 6 | 0 |
| Fistula | 1 | 0 | 0 | 1 | 0 | 0 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download