Abstract
Purpose
To evaluate the efficacy of hydromorphone-OROS (HM-OROS) in reducing sleep disturbance and relieving cancer pain.
Materials and Methods
One hundred twenty cancer patients with pain (numeric rating scale [NRS] ≥ 4) and sleep disturbance (NRS ≥ 4) were evaluated. The initial HM-OROS dosing was based on previous opioid dose (HM-OROS:oral morphine=1:5). Dose adjustment of the study drug was permitted at the investigator’s discretion. Pain intensity, number of breakthrough pain episodes, and quality of sleep were evaluated.
Results
A total of 120 patients received at least one dose of HM-OROS; 74 of them completed the final assessment. Compared to the previous opioids, HM-OROS reduced the average pain NRS from 5.3 to 4.1 (p < 0.01), worst pain NRS from 6.7 to 5.4 (p < 0.01), sleep disturbance NRS from 5.9 to 4.1 (p < 0.01), incidence of breakthrough pain at night from 2.63 to 1.53 times (p < 0.001), and immediate-release opioids use for the management of breakthrough pain from 0.83 to 0.39 times per night (p = 0.001). Of the 74 patients who completed the treatment, 83.7% indicated that they preferred HM-OROS to the previous medication. The adverse events (AEs) were somnolence, asthenia, constipation, dizziness, and nausea.
Cancer pain is one of the most distressing symptoms to cancer patients and family members [1-3]. Pain is not only burdensome, but it also worsens the quality of sleep [2,4,5]. Some studies have found that the prevalence of sleep disturbance in cancer patients ranges from 24% to 95% [5-7]. Insomnia of cancer patients is more common among females, older individuals, and those suffering from depression or anxiety [6-9]. Medical staff treating cancer patients should focus on controlling pain efficiently and increasing quality of sleep [6,10]. Opioids are useful for the initial restoration of nighttime sleep [6,10]. However, long-term opioid use can cause sedation and daytime sleeping, as well as disturbed sleep patterns and circadian rhythms [6,11-14]. Hydromorphone-OROS (HM-OROS) is gradually absorbed for 24 hours and does not cause fluctuations in blood concentration, unlike with short-acting hydromorphone [15-18]. This study was a multicenter, prospective, and open-labeled study. The primary objective is to evaluate the efficacy of HM-OROS in the treatment of sleep disturbance associated with cancer pain. The secondary objectives were to show the changes in Korean Brief Pain Inventory (K-BPI) during study drug administration and to evaluate participants’ preference for HM-OROS over the previous opioids [19].
This study enrolled adult patients (≥ 20 years of old) with cancer pain (numeric rating scale [NRS] ≥ 4) and sleep disturbance (NRS ≥ 4). Subjects have been administered with strong oral opioid analgesic for the purpose of cancer pain management. Exclusion criteria included evidence of cardiovascular, renal, hepatic, psychiatric, gastrointestinal disease or structural abnormalities, intolerance or hypersensitivity to hydromorphone, pregnancy, breastfeeding, and administration of monoamine oxidase inhibitor. The institutional review boards of the participating institutions approved this protocol, and written informed consents were obtained from all participants before entry into the study.
This study was conducted at 6 university hospitals in Korea, from September 2008 to February 2010. All hospitals had approval from their respective institutional review board. The primary objective is to evaluate the efficacy of HM-OROS in the treatment of sleep disturbance associated with cancer pain. This study consisted of 3 phases. The first phase was the screening phase (1 week) to evaluate the eligibility of potential subjects. The second phase (2 weeks) was to evaluate the efficacy and safety, and the third phase (12 weeks) involved extended follow-up for safety. Starting the first day of the second phase, participants took an equianalgesic dose of HM-OROS for two weeks. The dose of HM-OROS was determined based on the total dose of previous opioids that were administered on the last day of the screening phase [20]. If the dose of the previously administered opioids was appropriate (the number of short-acting opioid analgesic administrations for breakthrough pain treatment ≤ 3), the initial dose of the study drug was determined by an equivalent conversion ratio. If the dose of the previously administered long-acting oral opioids was not appropriate (the number of short-acting opioid analgesic administration for breakthrough pain treatment > 4), the higher dose of the study drug was set at the initial dose. When the dose of HM-OROS was ≤ 32 mg, we increased the first dose of HM-OROS by 8 mg, and if the dose was > 32 mg, we increased by 16 mg. We continuously adjusted the dose of the study drug every two days from day 3 of the second phase. We surveyed each subject’s pain intensity and short-acting opioid use for breakthrough pain. Sleep disturbance was evaluated at day 1 of the second phase and on the last day of the second phase. Pain was measured with the K-BPI, which consists of six parts: 1) presence of pain; 2) site of pain; 3) four-item pain score for the worst, least, and average pain during the previous 24 hours and at last evaluation; 4) type of pain medication; 5) percentage of pain relieved during the previous 24 hours; and 6) sevenitem associated symptom score, composed of interference with general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life [19]. To evaluate the preference to HM-OROS over that of the previous opioids, we conducted a preference questionnaire, including the reason for preference after the second phase. We also performed a clinical global impression-improvement survey of patients and physicians. Patients who completed phase 2 and suffered from continuing cancer pain were enrolled in the extension phase. Before the performance of any extension phase activity, the subjects were fully informed regarding this extension study and signed the written consent. During this extension phase, safety information, including adverse events, was collected.
The minimum sample size was 107, and the number of subjects in consideration of 20% of expected withdrawal rate was 134. This sample size provided approximately 80% power to detect a difference between HM-OROS and previous opioid for the primary efficacy analysis and a 2.5% type 1 error rate. Demographic information was collected from 190 patients who provided informed consent at the initial visit (Fig. 1). Safety assessment was evaluated from 120 patients who received the study drug at least once. Efficacy was assessed from 74 patients who completed the second phase (per protocol [PP] set), and 82 other patients (intentto-treat [ITT] set). In the ITT set, we included patients who took the study drug at least once, but excluded 38 patients who did not have major required variables or who committed serious protocol violations. Change from the baseline in pain score and sleep disturbance was tested using a paired t-test. The same analyses were also conducted for the subgroup of patients who maintained the same dose of the study drug during the study period. The proportion of sleep disturbance improvement and 95% confidence interval (CI) was calculated. To analyze the data for the secondary objectives of the study, Pearson’s chi-square test and multiple comparisons were conducted. The SAS program ver. 9.1 (SAS Institute Inc., Cary, NC) was used for all statistical analyses and the level of significance was 5%.
The baseline characteristics of the patient population are shown in Table 1. A total of 190 patients were enrolled in the study. However, only 120 patients received at least one dose of HM-OROS; of these 120, 74 completed the final assessment. Fig. 1 is a flow diagram of patient entry and completion, including the reasons of dropouts. The mean age was 56 years (standard deviation [SD], 11.2) and 129 (67.9%) of the patients were male (Table 1). All patients were diagnosed with cancer. The common primary diagnoses were colorectal (11%), lung (9.5%), pancreas (9%), stomach (8%), and breast (7%). The majority of these patients were stage IV (81.1%). The most common Eastern Cooperative Oncology Group performance statuses were 1 (68.9%) and 2 (24.3%). Seventyfive percent of patients had a history of previous treatment, including 121 cases of chemotherapy (63.7%), 30 cases of radiotherapy (15.8%), and 29 cases of surgical treatment (15.3%).
The ITT set was used as a primary population. At the second visit, we rotated previous opioids to HM-OROS at an equianalgesic dose (Fig. 1). The initial mean dose of HM-OROS was 22.6 mg (SD, 18.9; range 8 to 80 mg). At the third visit (endpoint of phase 2), the mean dose of HM-OROS was 30.2 mg (SD, 27; range, 8 to 112 mg). Forty patients (54.1%) maintained the same dose, while 19 patients (25.7%) needed dose escalation and 15 (20.3%) needed dose reduction. HM-OROS reduced the average pain score from 5.3 to 4.1 (p < 0.01), worst pain score from 6.7 to 5.4 (p < 0.01), sleep disturbance score from 5.9 to 4.1 (p < 0.01), incidence of breakthrough pain at night from 2.63 to 1.53 times (p < 0.001), and immediate-release opioids use for the management of breakthrough pain from 0.83 to 0.39 times per night (p=0.001). Table 2 shows the results for three questions regarding sleep disturbance. All three items improved during HM-OROS treatment. The mean proportion of improvement of sleep disturbance was 34.9% (95% CI, 27.4% to 41.9%), and 58.5% of the 82 (ITT) patients responded with greater than 30% improvement (Table 3). The result of K-BPI showed that pain, mood, walking ability, normal work, and sleep improved during the HM-OROS treatment (Table 4). In total, 57% of patients and 54% of physicians reported that HM-OROS was effective in global assessment (Table 5). The results from PP set were similar.
A total of 120 patients receiving HM-OROS were included in the safety analysis. Most of the adverse events were classified as either mild or moderate in intensity and were transitory. The most common adverse events were somnolence, asthenia, constipation, dizziness, and nausea (Table 6).
Only 2 cases (1 constipation, 1 dizziness) were severe, but were manageable. The constipation case was associated with the underlying disease (colon cancer) and the dizziness case was considered to be related to the study drug. None of the deaths was considered related to the study medication.
Of the patients who completed the treatment, 83.7% indicated that they preferred HM-OROS to the previous opioid. The reasons for this preference were as follows: decrease in medication frequency (53%), decrease in sleep disturbance (32%), and decrease in immediate release opioid use (13%).
In the present study, we have shown that the severity of pain and sleep disturbance decreased effectively during the HM-OROS treatment. Previous studies have reported pain improvement associated with HM-OROS. However, to the best of our knowledge, there are no reports on sleep disturbance improvement with the study drug in cancer patients [18,20-22]. Overall, the mean decreases in pain intensity and sleep disturbance were 18.2% and 34.9%, respectively. Stable drug concentration and decrease in the end-of-dose failure are considered major reasons for those results [23]. HM-OROS is a unique long-acting opioid formulation that maintains consistent hydromorphone plasma concentration, providing long-lasting analgesia. Using this delivery method, hydromorphone is steadily released, thereby allowing more constant pain control [23]. In global assessment, those who answered extremely effectively, very effectively, or effectively showed a good correlation coefficient with improvement of sleep disturbance (correlation coefficient=–0.57, p < 0.01). To exclude the effect of study drug dose change, we performed subgroup analysis with 40 patients (54.1%) who did not experience a change in the dose of HM-OROS. This analysis also showed that sleep disturbance improved by 10%.
A previous study reported that one of the major causes of suboptimal therapy outcomes was poor adherence to prescribed treatment regimens [23]. In addition to the reduction in peak-trough variability of HM-OROS compared with previous opioid, the simplified once-daily dosing regimen can potentially improve medication adherence. In interpreting this study’s findings, several limitations need to be taken into account. First, this study was not a randomized trial. Therefore, data should be confirmed in controlled studies with a larger patient population. Second, for better pain control, the adjustment of study drug dose was allowed at the discretion of the physician. This may be a weak point to evaluate the pure efficacy of HM-OROS, but additional analysis of patients who did not change the dose of study drug showed a similar finding. Third, a previous study showed that insomnia of advanced cancer patients was associated with multiple factors, including pain, depression, and fatigue. Therefore, it is difficult to determine the relationship between pain and sleep disturbance among study participants. Fourth, we had a dose-titration period of previous opioid for at least seven days. However, we are not sure that the last dose of previous opioid was optimal. Lastly, due to the nature of cancer pain studies, many seriously ill patients were enrolled. Therefore, many screen failures, protocol violations, and dropouts occurred. Despite of the aforementioned limitations, this study provides useful insights into the effectiveness of HM-OROS for the relief of cancer pain and improvement of associated sleep disturbance.
ACKNOWLEDGMENTS
We would like to thank all patients who agreed to participate in the research, as well as all the researchers for meticulous recording of results and fulfillment of protocols.
This research was supported financially by grants from the Janssen Pharmaceuticals, whose role was restricted to providing assistance to the investigators in the conception, conduct, and analysis of this study.
References
1. Oechsle K, Goerth K, Bokemeyer C, Mehnert A. Symptom burden in palliative care patients: perspectives of patients, their family caregivers, and their attending physicians. Support Care Cancer. 2013; 21:1955–62.

2. Coyle N, Adelhardt J, Foley KM, Portenoy RK. Character of terminal illness in the advanced cancer patient: pain and other symptoms during the last four weeks of life. J Pain Symptom Manage. 1990; 5:83–93.

3. Yamaguchi T, Shima Y, Morita T, Hosoya M, Matoba M; Japanese Society of Palliative Medicine. Clinical guideline for pharmacological management of cancer pain: the Japanese Society of Palliative Medicine recommendations. Jpn J Clin Oncol. 2013; 43:896–909.

4. Mercadante S, Prestia G, Ranieri M, Giarratano A, Casuccio A. Opioid use and effectiveness of its prescription at discharge in an acute pain relief and palliative care unit. Support Care Cancer. 2013; 21:1853–9.

5. Mercadante S, Girelli D, Casuccio A. Sleep disorders in advanced cancer patients: prevalence and factors associated. Support Care Cancer. 2004; 12:355–9.

6. Graci G. Pathogenesis and management of cancer-related insomnia. J Support Oncol. 2005; 3:349–59.
7. Vena C, Parker K, Cunningham M, Clark J, McMillan S. Sleep-wake disturbances in people with cancer part I: an overview of sleep, sleep regulation, and effects of disease and treatment. Oncol Nurs Forum. 2004; 31:735–46.

8. Sela RA, Watanabe S, Nekolaichuk CL. Sleep disturbances in palliative cancer patients attending a pain and symptom control clinic. Palliat Support Care. 2005; 3:23–31.

9. Akechi T, Okuyama T, Akizuki N, Shimizu K, Inagaki M, Fujimori M, et al. Associated and predictive factors of sleep disturbance in advanced cancer patients. Psychooncology. 2007; 16:888–94.

10. Kvale EA, Shuster JL. Sleep disturbance in supportive care of cancer: a review. J Palliat Med. 2006; 9:437–50.

11. Hearson B, Sawatzky JA. Sleep disturbance in patients with advanced cancer. Int J Palliat Nurs. 2008; 14:30–7.

12. Gibbins J, McCoubrie R, Kendrick AH, Senior-Smith G, Davies AN, Hanks GW. Sleep-wake disturbances in patients with advanced cancer and their family carers. J Pain Symptom Manage. 2009; 38:860–70.

13. Mystakidou K, Parpa E, Tsilika E, Gennatas C, Galanos A, Vlahos L. How is sleep quality affected by the psychological and symptom distress of advanced cancer patients? Palliat Med. 2009; 23:46–53.
14. Carney S, Koetters T, Cho M, West C, Paul SM, Dunn L, et al. Differences in sleep disturbance parameters between oncology outpatients and their family caregivers. J Clin Oncol. 2011; 29:1001–6.

15. Nalamachu SR, Kutch M, Hale ME. Safety and Tolerability of Once-Daily OROS((R)) hydromorphone extended-release in opioid-tolerant adults with moderate-to-severe chronic cancer and noncancer pain: pooled analysis of 11 clinical studies. J Pain Symptom Manage. 2012; 44:852–65.
16. Hanna M, Tuca A, Thipphawong J. An open-label, 1-year extension study of the long-term safety and efficacy of once-daily OROS(R) hydromorphone in patients with chronic cancer pain. BMC Palliat Care. 2009; 8:14.

17. Hanna M, Thipphawong J; 118 Study Group. A randomized, double-blind comparison of OROS(R) hydromorphone and controlled-release morphine for the control of chronic cancer pain. BMC Palliat Care. 2008; 7:17.

18. Wallace M, Rauck RL, Moulin D, Thipphawong J, Khanna S, Tudor IC. Once-daily OROS hydromorphone for the management of chronic nonmalignant pain: a dose-conversion and titration study. Int J Clin Pract. 2007; 61:1671–6.
19. Yun YH, Mendoza TR, Heo DS, Yoo T, Heo BY, Park HA, et al. Development of a cancer pain assessment tool in Korea: a validation study of a Korean version of the brief pain inventory. Oncology. 2004; 66:439–44.

20. Lawlor P, Turner K, Hanson J, Bruera E. Dose ratio between morphine and hydromorphone in patients with cancer pain: a retrospective study. Pain. 1997; 72:79–85.

21. Stepanovic A, Pirc J, Lahajnar Cavlovic S. Clinical efficacy of OROS(R) hydromorphone in patients suffering from severe chronic pain: A Study undertaken in routine clinical practice. Wien Klin Wochenschr. 2011; 123:531–5.
22. Bruera E, Sloan P, Mount B, Scott J, Suarez-Almazor M. A randomized, double-blind, double-dummy, crossover trial comparing the safety and efficacy of oral sustained-release hydromorphone with immediate-release hydromorphone in patients with cancer pain. Canadian Palliative Care Clinical Trials Group. J Clin Oncol. 1996; 14:1713–7.

23. Gardner-Nix J, Mercadante S. The role of OROS hydromorphone in the management of cancer pain. Pain Pract. 2010; 10:72–7.
Fig. 1.
Summary of study design and patient disposition. ITT, intent-to-treat; PP, per-protocol. a)±2 days.
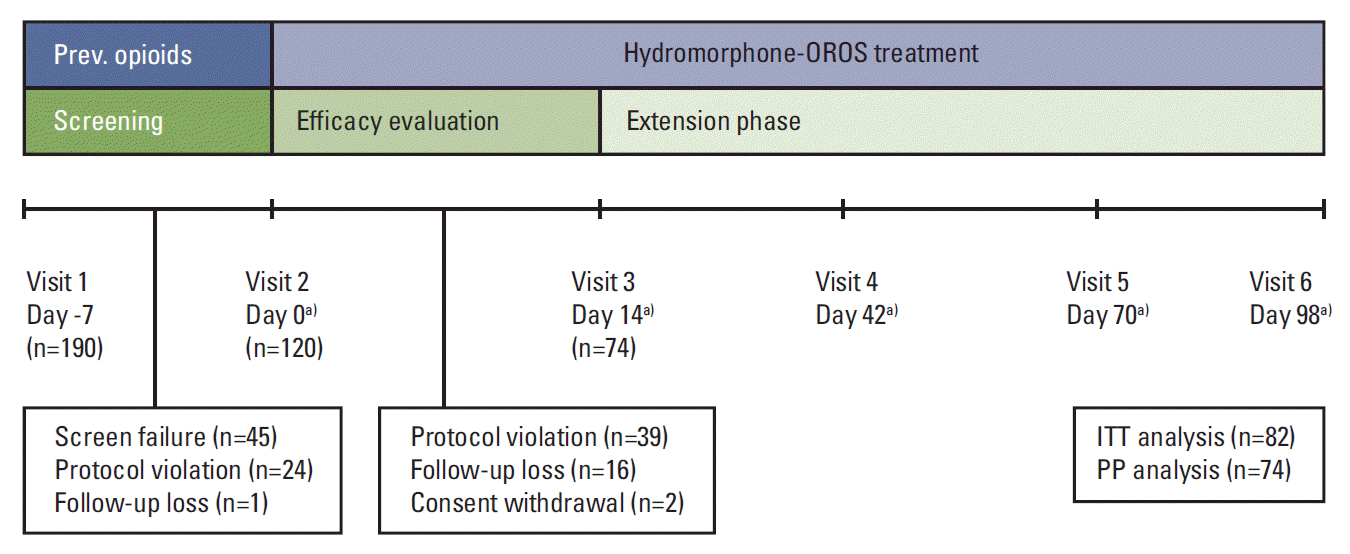
Table 1.
Patient characteristics (n=190)
Table 2.
Pain score and sleep disturbance score during hydromorphone-OROS treatment (n=82, intent-to-treat)
| Visit 2 | Visit 3 | p-value | |
|---|---|---|---|
| Pain score (NRS, mean士SD) | |||
| Worst NRS | 6.7±1.9 | 5.4 ±2.0 | < 0.01a) |
| Least NRS | 3.6 ±2.1 | 2.9±1.9 | < 0.01a) |
| Average NRS | 5.2 ±1.6 | 4.1±1.9 | < 0.01a) |
| Right now NRS | 4.4 ±1.8 | 3.7± 2.0 | < 0.01a) |
| Sleep disturbance (NRS) | 5.9 ±1.9 | 4.1 ±2.5 | < 0.01a) |
| Did you need to take an analgesic for | 45.1 | 30.5 | 0.02b) |
| pain relief in order to go to sleep last night? (yes, %) | |||
| How many times did you wake up | < 0.01c) | ||
| while sleeping late night? (%) | |||
| 0 | 0 | 0 | |
| 1 | 10.4 | 39.7 | |
| 2 | 37.7 | 29.3 | |
| 3 | 27.3 | 12.1 | |
| 4 | 7.8 | 12.1 | |
| > 5 | 16.9 | 6.9 | |
| Did you wake up in the morning | < 0.01b) | ||
| because of unbearable pain? (yes, %) | 39 | 18.3 |
Table 3.
Improvement in sleep disturbance during hydromorphone-OROS treatment (n=82, intent-to-treat)
| No. of patients (%) | 95% CI | p-valuea) | |
|---|---|---|---|
| Mean proportion of sleep disturbance improvement | 29 (34.9) | 27.8-41.9 | - |
| Greater than 10% improvement | 63 (85.1) | 74.8-91.1 | < 0.01 |
| Greater than 30% improvement | 48 (58.5) | 47.9-69.2 | < 0.01 |
| Greater than 50% improvement | 22 (26.8) | 17.2-36.4 | < 0.01 |
Table 4.
Korean brief pain inventory during hydromorphone-OROS treatment (n=82, intent-to-treat)
|
Visit 2 |
Visit 3 |
p-valuea) | |||
|---|---|---|---|---|---|
| No. | Mean 土 SD | No. | Mean 土 SD | ||
| Rate your pain at its worst in the last week | 74 | 6.7±1.9 | 75 | 5.4±2.0 | < 0.01 |
| Rate your pain at its least in the last week | 74 | 3.6±2.1 | 75 | 2.9±1.9 | < 0.01 |
| Rate your pain on average in the last week | 74 | 5.2±1.6 | 74 | 4.1±1.9 | < 0.01 |
| Rate your pain right now | 76 | 4.4±1.8 | 75 | 3.7±2.0 | < 0.01 |
| Generally activity | 76 | 5.0±2.0 | 75 | 5.0±2.0 | 0.19 |
| Mood | 76 | 5.1±2.0 | 75 | 4.6±2.3 | 0.04 |
| Walking ability | 75 | 5.0±2.3 | 75 | 4.5±2.6 | 0.01 |
| Normal work | 76 | 5.6±2.2 | 75 | 4.9±2.4 | 0.01 |
| Relations with other people | 76 | 4.8±2.6 | 75 | 4.6±2.7 | 0.35 |
| Sleep | 76 | 5.9±1.8 | 74 | 4.2±2.5 | < 0.01 |
| Enjoyment of life | 76 | 5.5±2.5 | 75 | 5.0±2.6 | 0.09 |
Table 5.
Global assessment of patients and physic ians (n=74, per protocol)
| Patient (%) | Physician (%) | |
|---|---|---|
| Extremely effective | 0 (0) | 1 (1.4) |
| Very effective | 8 (10.8) | 12 (13.5) |
| Effective | 34 (46) | 29 (39.2) |
| Fair | 25 (33.8) | 28 (37.8) |
| Not effective | 7 (9.5) | 6 (8.1) |
Table 6.
Adverse events most commonly reported by ≥ 5% patients (n=120)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download