Abstract
Purpose
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) represent a heterogeneous disease group originating from the neuroendocrine cells. Identification of prognostic markers, related to neuroendocrine tissue-selective tumorigenesis, is necessary to find therapeutic targets.
Materials and Methods
A total of 327 patients with GEP-NETs were included in this study; there were 49 gastric, 29 duodenal, 49 pancreatic, 12 hepatobiliary, 33 appendiceal, 5 proximal colon, and 150 distal colon cases. We performed immunostaining with the tissue microarray method for menin, p27, and p18.
Results
We observed negative staining for menin, p27, and p18 in 34%, 21%, and 56% of GEP-NETs, respectively. The loss of p27, but not menin, was positively correlated with the grade of Ki-67. Menin–/p27–, menin–/p27+, menin+/p27–, and menin+/p27+ phenotype groups included 13%, 22%, 8%, and 57% of patients, respectively. A dichotomized comparison showed that menin– or p27– tumors were significantly associated with foregut and midgut localizations, high World Health Organization (WHO) grade, lymph node metastasis, and more advanced stage as compared to menin+/p27+ patients. Kaplan-Meier analysis for the overall survival showed that p27 loss was significantly associated with decreased survival. Multivariate analysis showed that p27 loss is an independent factor for poor overall survival.
Gastroenteropancreatic neuroendocrine tumors (GEPNETs) comprise 65-75% of all NETs and arise from the enterochromaffin cells distributed throughout the digestive system. The prevalence of GEP-NETs has been estimated to be 2.23/100,000, with an annual onset incidence of 1.01/100,000 [1]. The incidence and prevalence of this kind of tumor have increased over the recent decades, according to a recent analysis of the Surveillance, Epidemiology, and End Results (SEER) Program database in the United States [2]. Although most NETs present indolent progression, a significant number of patients were diagnosed presenting with unresectable or metastatic disease.
The MEN1 gene is located on chromosome 11q13 and consists of 10 exons that encode a protein of 610 amino acids, referred to as menin [3]. Mutation in the MEN1 gene has been identified in approximately 90% of familial cases and 27% of sporadic cases of MEN1 syndrome. More than 500 different somatic and germline MEN1 gene mutations have been identified; however, no obvious genotype-phenotype correlation is discernible with these mutations [4]. Based on both reverse transcription polymerase chain reaction and immunoblotting, menin expression was reported to be down-regulated in MEN1 tumors [5]. Menin has functions in DNA stability and gene regulation, and it can act as a tumor suppressor [6]. It has been shown to function as a tumor suppressor through transcriptional activation of the cyclin-dependent kinase (CDK) inhibitors, p18 and p27 [7]. Loss of menin was conversely associated with the reduction of both p18 and p27 gene expression [8]. Menin has been shown to bind to the promoters of p18 and p27 together with mixed lineage leukemia protein (MLL) histone methyltransferase in mouse pancreatic islets [7,8].
p27 is encoded by cyclin-dependent kinase inhibitor 1B (CDKN1B), and inhibitory binding of p27 to CDK2/cyclin E and CDK2/cyclin A complexes in the nucleus arrests cells at G1/S in the cell cycle [9]. Low expression of p27 has been observed in about 50% of all human cancers and this condition is usually correlated with histological aggressiveness and poor outcome in patients with breast, colorectal, ovary, prostate, bladder, and pancreatic tumors [10-12]. Loss of p27 expression was also found in endocrine neoplasms, such as human pituitary and parathyroid hyperplasias, adenomas, sporadic pheochromocytoma, as well as GEPNETs [12-15].
A mutation in the p27 gene was recently identified in a family with what appeared to be MEN1-related tumors, which have now been proposed to be called MEN type 4 (MEN4) [9]. p27-null mice develop intermediate lobe pituitary adenomas as the sole tumor phenotype, suggesting that pituitary cells were particularly sensitive to the defects in cell cycle regulation [9]. That finding suggested that p27 is associated with tumorigenesis of the neuroendocrine cells. Since the growth of mouse endocrine organs is sensitive to simultaneous loss of p27 and p18 activities [16,17], it was suggested that p27 and p18 may have partially overlapping functions in the maintenance of growth control for diverse neuroendocrine cells.
Up until now, only a few studies have focused on the loss of menin, p27, and p18 in GEP-NETs [13,14,18], and the clinical significance of the loss of these proteins has been controversial. We hypothesized that there is a tissue-specific tumorigenesis pathway that involves menin protein alteration with subsequent p27 loss in GEP-NETs. Herein, we examine the GEP-NET expression of menin, p27, and p18 [19].
The medical records of 327 patients (mean age, 53 years; range, 11 to 91 years) with histopathologically proven neuroendocrine tumors of the gastroenteropancreatic tract, who were treated at Seoul National University Hospital (n=230) or Seoul National University Bundang Hospital (n=97) between 1989 and 2009, were analyzed retrospectively.
Histopathologic confirmation of the diagnosis of GEP-NET was required to be included in this study. The patient files were reviewed systematically for the date of initial diagnosis, localization of the primary tumor, histopathologic diagnosis, tissue site from which samples were taken for histopatho-logic diagnosis, clinical staging at initial diagnosis, and the presence of a functional syndrome (Table 1). There were 145 female and 182 male patients. A family history of the disease was present in seven patients. Twenty-six patients had symptoms at presentation. Five patients had endocrine disorders. Standard histopathological examinations included assessment of the pathological tumor stage according to the criteria described in the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual [20]. Histologic classifications were performed as recommended by the World Health Organization (WHO) 2010 classification [21].
The formalin-fixed and paraffin-embedded tumor tissues of patients treated from 1989 to 2008 were retrieved from the archives at the Departments of Pathology of Seoul National University Hospitals in Seoul and Bundang, Korea. The inclusion criteria were primary GEP-NET with or without metastases diagnosed by endoscopic biopsy and surgical resection.
Among the 327 patients, follow-up and survival information was obtained for 262 patients. The mean follow-up period was 53 months (range, 1 to 243 months), as of February 2012. The study protocol was approved by the Institutional Review Board committee of Seoul National University Hospital (C-1012-027-343). Patient survival data, including dates and causes of death, were obtained from the Korean Central Cancer Registry at the Ministry of Health and Welfare.
Fifty-two patients received adjuvant treatments, including postoperative chemotherapy (n=25), postoperative chemotherapy/radio frequency ablation (RFA)/sandostatin longacting release (LAR) (n=1), preoperative chemotherapy (n=2), preoperative and postoperative chemotherapy (n=1), postoperative radiotherapy (RT) and chemotherapy (n=7), RT (n=2), RFA (n=4), transarterial chemoembolization (TACE) (n=4), transarterial embolization (TAE) and RT (n=1), TACE and chemotherapy (n=1), TACE and RAD001 trial (n=1), TACE+RFA+RAD001 trial (n=1), TAE+ interferon+streptomycin+adriamycin, sandostatin+LAR+ thalidomide+Avastin+RAD001 (n=1), and TACE+RFA+RT+ sandostatin+chemotherapy (n=1).
Tissues obtained from patients were routinely fixed in 10% buffered formalin and embedded in paraffin blocks. After screening the available slides for each case, we selected a paraffin block that was well fixed and contained a representative section of the tumor. One tissue column (2.0 mm in diameter) was obtained from each selected paraffin block and arranged in new, separate paraffin blocks with 60 holes, using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea). Microarray blocks were then sectioned at 4 μm and processed for immunohistochemical staining. After removing the paraffin with xylene, the sections were rehydrated with graded ethanol and immersed in Tris-buffered saline.
Anti-menin rabbit monoclonal (1:200, Epitomics, Burlingame, CA), p18 mouse monoclonal (1:50, Thermo Scientific, San Jose, CA), and p27 rabbit polyclonal (1:300, Spring Bioscience, Pleasanton, CA) antibodies were used for immunohistochemistry. Staining was done by a labeled avidin-biotin-peroxidase complex, using the ABC kit from Vector Labs (Vectastain Elite ABC kit, Vector Labs, Burlingame, CA) after antigen retrieval. For color development, diaminobenzidine was used. The nuclear Ki-67 labeling index was expressed as the percentage of positively stained cells with respect to 100 cells in 10 high-power fields. For p27 immunostaining, the nuclear staining and cytoplasmic staining were evaluated separately. Cases with no staining of either cytoplasm or nucleus were considered to be negative. p18 was regarded as positive when nuclear staining was present in more than 10% of the cells. Menin staining was regarded as positive when more than 10% of the cells had positive staining, in either the nuclei or cytoplasm.
Our series contains statistical limitations because of heterogeneity among the primary sites and treatment modalities, as well as the 20-year collection period. Survival rates were calculated using the Kaplan-Meier method, and groups were compared using the log rank test. Kaplan-Meier curves were plotted using the overall survival (OS) data. Multivariate Cox regression analysis with variables including Ki-67 grade, presence of lymph node metastasis, p27-positivity, and menin-positivity was performed. All p < 0.05 were considered statistically significant. Statistical analysis was performed using the SPSS ver. 15.0 (SPSS Inc., Chicago, IL).
Staining of menin, p27, and p18 was evident in the nuclei of the normal pancreatic acini, ducts, and islet cells. Menin loss was observed in 34% (100/294) of the GEP-NET cells. Frequencies of p27-negativity and -positivity were 21% (61/290) and 79% (229/290; 70% nuclear positivity and 9% cytoplasmic positivity), respectively. p18-negativity was observed in 56% of cells (169/300) (Fig. 1).
Menin-negativity was more frequent in the foregut and pancreas, and in AJCC stages higher than II. p27-negativity was more frequent in the foregut or midgut locations, in higher grades by WHO classification, and in higher AJCC stages (Table 2).
To see the relationship between menin and p27 expression levels, patients were categorized into four groups in accordance to a combination of menin and p27 expression. Menin–/p27–, menin–/p27+, menin+/p27–, and menin+/p27+ phenotype groups, which included 13% (37/290), 22% (63/290), 8% (24/290), and 57% (166/290) of patients, respectively. Among 61 p27– cases, 37 (61%) were menin–, while among 229 p27+ cases, 63 (28%) were menin–.
We performed comparative analyses among the 4 groups of menin+/p27+, menin–/p27–, menin–/p27+, and menin+/p27–. The tumors of the foregut or midgut had a greater loss of either menin or p27; whereas the menin+/p27+ phenotype were more frequent in hindgut tumors (p=0.001). In the classification of the GEP-NET cases into gastrointestinal and pancreatic NETs, pancreatic NETs were less frequently menin+/p27+ than were gastrointestinal NETs (44% vs. 60%, respectively), although this difference was not statistically significant. Tumors defined as low grade by the WHO classification (NET G1 and G2) (p=0.021), those that were AJCC stage I (p=0.002), and those without lymph node metastasis (p=0.002) were more frequently found in the menin+/p27+ phenotype group than in the other groups (Table 3).
The correlation of p27 expression with the expression levels of other relevant proteins was analyzed. The tumor proliferative index assessed using Ki-67 immunohistochemistry has been considered as a standard marker for therapeutic decisions in NETs. p27 loss was correlated with high grade Ki-67, but menin loss did not. Neither p27 expression nor menin expression was significantly associated with p18 expression (Table 4).
The Kaplan-Meier analysis for the OS showed that menin+ patients had better rates of survival than menin– patients, but with borderline significance (p=0.055); p27+ patients had a significantly better rate of survival than p27– patients (p < 0.001). In subgroup analyses with the combination phenotypes in accordance to p27 and menin expression, menin+/p27+ showed the best OS, both among the 4 groups and 2 dichotomized groups (p < 0.001) (Fig. 2). In a multivariate analysis, including the variables of Ki-67 grade, lymph node status, p27 expression, and menin expression, high grade Ki-67, lymph node metastasis, and p27-negativity were associated with significantly worse OS (Table 5).
Our study showed a loss of menin, p27, and p18 in 34%, 21%, and 56%, respectively, of GEP-NETs, and also found that the expression levels of those proteins are associated with the prognosis. In an analysis of the MEN1 gene and protein status in sporadic pancreatic endocrine neoplasms [22], somatic mutation of the MEN1 gene was detected in 15% of pancreatic NETs (25 of 169), whereas abnormal expression of menin was found in 80% of sporadic pancreatic NETs. Menin was also abnormally expressed in a significant number of NET cases lacking MEN1 mutations. These findings suggest that epigenetic regulation of the MEN1 gene plays a role in the tumorigenesis of neuroendocrine tumors [8].
Menin was found to be a part of a distinct MLL complex that contains histone methyl transferase (HMT) activity. This MLL-HMT complex specifically promotes the trimethylation of lysine 4 on histone H3 (H3K4) and, in such way, activates the transcription of homeobox domain (HOX) genes, p18 and p27 [8]. p18 and p27 are known to inhibit functions of the cell cycle regulators, like CDK2 and CDK4 [19]. The loss of function of menin leads to the down-regulation of p18 and p27, causing a deregulation of cell growth [7].
We observed a correlation of p27 loss with high Ki-67 grade and shorter overall survival, a finding that is consistent with previous results. p27 was investigated in endocrine tumors and a marked decrease was reported in the protein expression of hyperplastic tissues, as well as benign and malignant neoplasms compared with normal tissues [12]. Grabowski et al. [14] reported a loss of nuclear p27 expression and its prognostic role in relation to cyclin E and p53 mutations in GEP-NETs. In pheochromocytoma, p27 expression was reduced or lost in 56% of tumors [13].
A limitation of our study is that our cases were retrospectively collected and were composed of a heterogeneous patient group with seven primary sites, various pathologic stages, and different treatment modalities. In addition, as the cases were collected through 20 years of pathologic archives, survival analyses may be somewhat skewed and could be influenced by compounding effects.
A strength of our study is that we analyzed 327 patients with GEP-NETs, making our study the largest among all those ever published on tissue-based GEP-NET studies [14,18]. In addition, we are the first to analyze the interactions among menin, p27, and p18 proteins in a large clinical cohort of GEP-NETs. Our study showed that menin loss was also present in a substantial portion of the p27 loss cases, although the underlying molecular defects related to menin protein loss were not elucidated in this study. Further investigation of p27 dysregulation with regard to the steps of NET progression is needed.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2012R1A1A2004648).
References
1. Gastrointestinal Pathology Study Group of Korean Society of Pathologists, Cho MY, Sohn JH, Jin SY, Kim H, Jung ES, et al. Proposal for a standardized pathology report of gastroenteropancreatic neuroendocrine tumors: prognostic significance of pathological parameters. Korean J Pathol. 2013; 47:227–37.

2. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26:3063–72.

3. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997; 276:404–7.

4. Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008; 29:22–32.

5. Bhuiyan MM, Sato M, Murao K, Imachi H, Namihira H, Takahara J. Expression of menin in parathyroid tumors. J Clin Endocrinol Metab. 2000; 85:2615–9.

6. Yang Y, Hua X. In search of tumor suppressing functions of menin. Mol Cell Endocrinol. 2007; 265-266:34–41.

7. Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005; 102:749–54.

8. Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A. 2005; 102:14659–64.

9. Marinoni I, Pellegata NS. p27kip1: a new multiple endocrine neoplasia gene? Neuroendocrinology. 2011; 93:19–28.

10. Duncan TJ, Al-Attar A, Rolland P, Harper S, Spendlove I, Durrant LG. Cytoplasmic p27 expression is an independent prognostic factor in ovarian cancer. Int J Gynecol Pathol. 2010; 29:8–18.

11. Psyrri A, Bamias A, Yu Z, Weinberger PM, Kassar M, Markakis S, et al. Subcellular localization and protein levels of cyclin-dependent kinase inhibitor p27 independently predict for survival in epithelial ovarian cancer. Clin Cancer Res. 2005; 11:8384–90.

12. Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, et al. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999; 154:313–23.

13. Pellegata NS, Quintanilla-Martinez L, Keller G, Liyanarachchi S, Hofler H, Atkinson MJ, et al. Human pheochromocytomas show reduced p27Kip1 expression that is not associated with somatic gene mutations and rarely with deletions. Virchows Arch. 2007; 451:37–46.

14. Grabowski P, Schrader J, Wagner J, Horsch D, Arnold R, Arnold CN, et al. Loss of nuclear p27 expression and its prognostic role in relation to cyclin E and p53 mutation in gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res. 2008; 14:7378–84.

15. Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Hofler H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A. 2006; 103:15558–63.

16. Franklin DS, Godfrey VL, O'Brien DA, Deng C, Xiong Y. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol. 2000; 20:6147–58.

17. Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S, et al. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998; 12:2899–911.

18. Rahman A, Maitra A, Ashfaq R, Yeo CJ, Cameron JL, Hansel DE. Loss of p27 nuclear expression in a prognostically favorable subset of well-differentiated pancreatic endocrine neoplasms. Am J Clin Pathol. 2003; 120:685–90.

19. Cavallari I, D'Agostino DM, Ferro T, Rosato A, Barzon L, Pasquali C, et al. In situ analysis of human menin in normal and neoplastic pancreatic tissues: evidence for differential expression in exocrine and endocrine cells. J Clin Endocrinol Metab. 2003; 88:3893–901.
20. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag;2009. p. 181–9.
21. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Lyon: IARC Press;2010. p. 13–4.
Fig. 1.
Immunohistochemical expression of menin, p27, and p18 in gastroenteropancreatic neuroendocrine tumors. (A, D, G) menin. (B, E, H) p27. (C, F, I) p18. Upper, gastric neuroendocrine carcinoma with 90% of Ki-67; middle, gastric neuroendocrine tumor with 1% of Ki-67, lower, rectal neuroendocrine tumor with 3% of Ki-67.
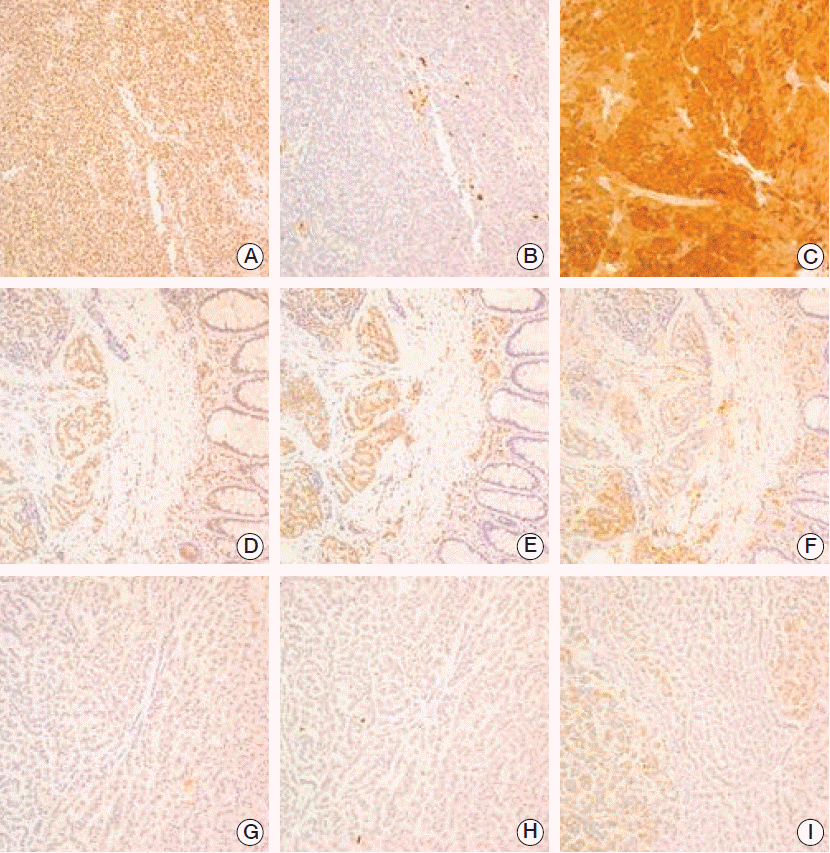
Fig. 2.
Kaplan-Meier analysis of overall survival in gastroenteropancreatic neuroendocrine tumors. Survival curves according to p27 expression (A), menin expression (B), 4 phenotype groups by combination of menin and p27 expression (C), and dichotomized groups of menin+/p27+ and menin– or p27– (D).
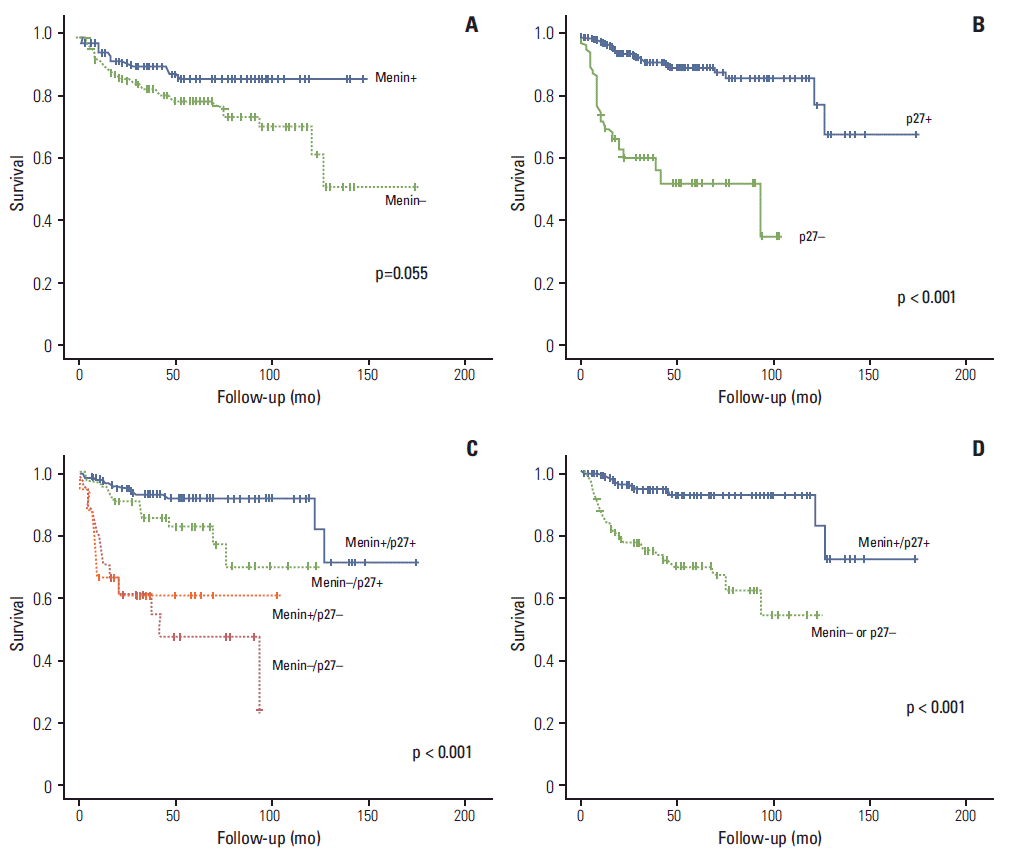
Table 1.
Profile of patients with gastroenteropancreatic neuroendocrine tumors
Table 2.
Clinicopathologic correlations of p27 and menin expressions in gastroenteropancreatic neuroendocrine tumors
Table 3.
Clinicopathologic correlations of combined menin and p27 protein expressions in gastroenteropancreatic neuroendocrine tumors
Table 4.
Correlation of menin and p27 expression with expression of other proteins in gastroenteropancreatic neuroendocrine tumors
Table 5.
Multivariate analysis for overall survival




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download