Abstract
Pemetrexed is approved as a first-line treatment for advanced non-squamous non-small cell lung cancer (NSCLC) with cisplatin and as a single agent for second-line treatment or for patients who show no disease progression after four cycles of platinum-based doublet induction chemotherapy as maintenance therapy. Pemetrexed has a modest toxicity profile and has not traditionally been regarded as a cause of interstitial pneumonitis. Here, we report on a rare case of pemetrexed-induced pneumonitis in a patient with NSCLC.
Pemetrexed is a potent inhibitor of thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase. The US Food and Drug Administration approved pemetrexed as a single agent or in association with cisplatin as a treatment for patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) [1,2]. Pemetrexed has been approved for maintenance treatment in patients with locally advanced or metastatic nonsquamous NSCLC whose disease has not shown progression after four cycles of platinum-based doublet induction chemotherapy [3]. Pemetrexed has a modest toxicity profile and has not traditionally been regarded as a cause of interstitial pneumonitis. We encountered a case of pemetrexed-induced pneumonitis in a patient with NSCLC.
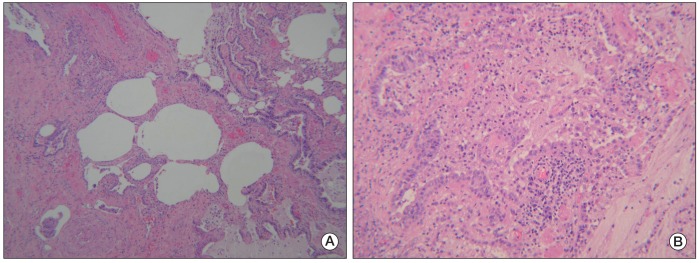
A 69-year-old male was admitted with fever and dyspnea. Seven months before admission, he was diagnosed with stage IV lung adenocarcinoma (cT3N2M1 for metastases of bone). He was an ex-smoker (45 packs/yr), and his performance status was Eastern Cooperative Oncology Group (ECOG) 1. He had no history of drug allergies. He was treated with first-line chemotherapy, including gemcitabine (1,000 mg/m2, days 1 and 8) and carboplatin (area under the curve, 5.5), every three weeks for six cycles. After four weeks from the last gemcitabine and carboplatin administration, disease progression was noted. The patient started second-line treatment with pemetrexed (500 mg/m2, day 1, every three weeks) with vitamin supplementation and steroid pre-medication. On admission, his vital signs were as follows: blood pressure, 110/70 mm Hg; pulse rate, 74/min; respiration rate, 24/min; and body temperature, 36.8℃. No abnormal heart sounds were detected on chest auscultation; however, fine crackles were detected in the bilateral lower lung fields. Results of arterial blood gas analysis showed severe hypoxia (pH, 7.470; pCO2, 28.2 mm Hg; pO2, 39.5 mm Hg; HCO3-, 20.7 mM; and O2 saturation, 84.6%); thus, the patient received 4 L/min oxygen through a nasal cannula. Peripheral blood tests showed the following: white blood cells, 7,600/µL; hemoglobin, 11.0 g/dL; and platelets, 170,000/µL. The patient's C-reactive protein concentration increased to 3.991 mg/dL, and routine chemistry showed the following: Na, 135 mM; K, 4.1 mM; Cl, 103 mM; aspartate transaminase, 26 IU/L; alanine aminotransferase, 17 IU/L; alkaline phosphatase, 113 IU/L; gamma glutamyl transferase, 29 IU/L; total bilirubin, 0.8 mg/dL; blood urea nitrogen, 8.8 mg/dL; and creatinine, 0.6 mg/dL. Lactic dehydrogenase increased to 1,094 IU/L. Findings on chest radiography revealed interstitial infiltration in both lung fields. Findings on computed tomography (CT) revealed diffuse ground glass opacities (Fig. 1A). Pneumococcal antigenuria and serology against Mycoplasma were negative. Due to the patient's immunocompromised status, treatment with broad-spectrum antibiotics (levofloxacin) and trimethoprim-sulfamethoxazole against Pneumocystis jiroveci was started. Bronchoscopy showed only anthracotic pigmentation and no endobronchial lesions. A microbiological culture obtained from bronchoalveolar lavage fluid showed no evidence of bacterial, viral, or fungal pathogens. Sputum cultures, acid-fast staining for bacilli, blood cultures, and P. jiroveci pneumonia polymerase chain reaction all showed negative results. An extensive microbiological investigation, including bronchoscopy, excluded the possibility of infection, including opportunistic infections. Treatment with pemetrexed, which was suspected as the cause of the drug-induced pneumonitis, was stopped, and high-dose prednisolone was administered. We performed a tissue biopsy via video-assisted thoracoscopic surgery in order to confirm interstitial pneumonitis. Markedly distorted lung architecture with interstitial collagenous fibrosis was observed on microscopic examination. Focal honeycomb changes were observed (Fig. 2A). The alveolar spaces were filled with luminal mucoid secretions and inflammatory cells. Chronic interstitial inflammation with interstitial fibroblastic activity was also observed (Fig. 2B). The pathological findings were compatible with nonspecific interstitial pneumonia. A diagnosis of drug-induced interstitial pneumonitis caused by pemetrexed was ultimately made; in response, antibiotic therapy was discontinued, and high-dose prednisolone therapy was continued for two weeks. Treatment with oral prednisolone resulted in improvement of the patient's clinical presentation and interstitial infiltration in both lung fields (Fig. 1B). The patient received third-line erlotinib therapy following his recovery from interstitial pneumonitis.
Here, we report on a case of pemetrexed-induced pneumonitis in a patient with NSCLC. Pemetrexed has a modest toxicity profile and is frequently used in patients with locally advanced or metastatic NSCLC. The most frequently reported pemetrexed-related toxicity was fatigue; other adverse events were less frequent. Clinical studies of pemetrexed have reported a lack of patients with grade 3-4 pulmonary toxicity [1-4]. One phase II study (n=244) reported eight cases, including one death from pneumonitis without definite clinical data, and three cases occurred in the arm that received 1,000 mg/m2 pemetrexed, which is greater than the usual 500 mg/m2 dosage [5]. A separate case report confirmed that pemetrexed can induce acute lung injury [6].
Diagnosis of drug-associated interstitial lung disease (ILD) is generally difficult. Such diagnoses are based on clinical suspicion, CT findings, and histological patterns [5]. Symptoms of drug-associated ILD, as with all forms of the condition, include rapidly developing breathlessness and a dry and unproductive cough, together with fever. Such symptoms are nonspecific and can occur with a large number of common illnesses often associated with NSCLC. These patients are also prone to pneumonia. The onset of drug-associated ILD during therapy for treatment of advanced NSCLC usually occurs within a few weeks after the start of treatment. Our patient presented with acute dyspnea and pulmonary infiltration on chest X-ray after three weeks of first-cycle pemetrexed treatment without evidence of an infectious cause. The time interval between the last gemcitabine cycle and the first pemetrexed cycle was four weeks, and no lesion indicative of penumonitis was observed on CT scans taken before the start of pemetrexed chemotherapy, therefore, the possibility of gemcitabine-related pneumonitis was low. No specific parenchymal change in the radiological pattern is observed with drug-associated ILD; however, common findings include the presence of ground-glass shadows and/or linear opacities on high-resolution CT (HRCT). It seemed reasonable to approach the radiological manifestations of drug-associated lung disease using the underlying histological pattern. We performed a tissue biopsy in order to prove the diagnosis pathologically. The patient's tissue showed the nonspecific interstitial pneumonia pattern of idiopathic interstitial pneumonia, according to the clinical-radiological-pathological diagnosis classification. Nonspecific interstitial pneumonia is one of the most common forms of drug-associated pneumonitis [7]. Nonspecific interstitial pneumonia is characterized histologically by homogeneous alveolar wall thickening with fibrous tissue and the presence of mononuclear inflammatory cells. This reaction is observed in association with methotrexate, amiodarone, and carmustine treatment. Corresponding radiographical and HRCT findings usually consist of patchy or diffuse ground-glass opacities. As the disease progresses, there may be evidence of fibrosis with development of a reticular pattern and traction bronchiectasis.
Drug-induced injury to the lungs is thought to result from either a direct toxic effect or an indirect effect by way of a hypersensitivity reaction. Anticancer agents such as bleomycin, methotrexate, procarbazine hydrochloride, and paclitaxel can cause pulmonary opacities as part of a hyper-sensitivity reaction; however, the pathophysiology of pemetrexed-related pneumonitis is unknown. In many instances, chemotherapy-associated lung disease may respond to withdrawal of the offending agent and the judicious application of corticosteroid therapy.
In summary, we report on a rare case of pemetrexed-induced pneumonitis in a patient with NSCLC, including radiological and pathological documentation. Diagnosis of chemotherapy-associated lung disease remains an exclusionary process, particularly with respect to infections and recurrence of the underlying neoplastic process in immuno-compromised patients. Given the increasing use of pemetrexed for treatment of lung cancer, physicians must be aware of this potential toxicity.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (no. 1020420).
References
1. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–3551. PMID: 18506025.

2. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–1597. PMID: 15117980.

3. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009; 374:1432–1440. PMID: 19767093.

4. Sun JM, Lee KW, Kim JH, Kim YJ, Yoon HI, Lee JH, et al. Efficacy and toxicity of pemetrexed as a third-line treatment for non-small cell lung cancer. Jpn J Clin Oncol. 2009; 39:27–32. PMID: 18952704.

5. Ohe Y, Ichinose Y, Nakagawa K, Tamura T, Kubota K, Yamamoto N, et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin Cancer Res. 2008; 14:4206–4212. PMID: 18594001.

6. Kim HO, Lee SY, Shim JJ, Kang KH, Shin BK. A case of pemetrexed-induced acute lung injury in non-small cell lung cancer. J Thorac Oncol. 2010; 5:401–402. PMID: 20186027.

7. Muller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer. 2004; 91(Suppl 2):S24–S30. PMID: 15340375.

Fig. 1
Chest computed tomography at presentation of interstitial pneumonitis (A) and after clinical improvement (B).

Fig. 2
(A) Microscopic examination of the lung specimen revealed distorted lung architecture with interstitial fibrosis and focal honeycomb changes (H&E staining, ×40). (B) The alveoli spaces were filled with luminal mucoid secretions with inflammatory cells. Chronic interstitial inflammation with interstitial fibroblastic activity was also observed (H&E staining, ×200).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download