Abstract
Purpose
There are three types of bile duct cancer, intrahepatic cholangiocarcinoma (ICC), hilar cholangiocarcinoma (HC), and extrahepatic cholangiocarcinoma (EHC). Despite different clinical presentation, the same protocol has been used in treatment of patients with these cancers. We analyzed clinicopathologic findings and protein expression in order to investigate the difference and the specific prognostic factors among these three types of cancers.
Materials and Methods
We conducted a retrospective review of 104 patients diagnosed with bile duct cancer at Seoul St. Mary's Hospital between January 1994 and May 2004. We performed immunohistochemical staining for p53, cyclin D1, thymidine phosphorylase, survivin, and excision repair cross-complementing group 1 (ERCC1).
Results
Of the 104 patients, EHC was most common (44.2%). In pathologic findings, perineural invasion was significantly less common in ICC. Overall survival was similar among the three types of cancer. Lymph node invasion, lymphatic, and venous invasion showed a significant association with survival outcome in ICC, however, the differentiation of histologic grade had prognostic significance in HC and EHC. No difference in protein expression was observed among these types of cancer, however, ERCC1 showed a significant association with survival outcome in HC and EHC, not in ICC.
There are three types of bile duct cancers (BDCs) according to the anatomical location; these include intrahepatic cholangiocarcinoma (ICC), hilar cholangiocarcinoma (HC), and extrahepatic cholangiocarcinoma (EHC). ICC is defined as a tumor originating from the intrahepatic duct, forming a liver mass; HC is defined as a tumor located in the bifurcation of the right and left bile duct, and EHC is defined as a tumor originating from the distal part of the bile duct below the hilar region. These cancers originally arise from the same bile duct; however, they have a few different clinical characteristics. For example, ICC, known as peripheral cholangiocarcinoma, usually presents with asymptomatic features, along with a hepatic mass like lesion, however, it less commonly shows obstructive symptoms or cholangitis, compared with the other two types of cancer [1]. Some modified staging systems showing correlation with hepatic resectability are used for ICC; however, because there is no unique staging system for BDC, tumor-node-metastasis (TNM) by the American Joint Committee on Cancer (AJCC) staging system is applied for EHC [2]. Some authors have reported that HC and ICC have different prognostic factors. The grade of the tumor cell histology and the level of bilirubin are important factors in HC, however, the presence of daughter nodules, and the serum level of carcinoembryonic antigen, the state of the resection margin, and the number of transfusions are important factors in ICC, and the pattern of KIT expression is important in EHC [3-6].
p53 expression has been commonly observed in BDC as well as in gastrointestinal (GI) adenocarcinoma, with a range of 25-85% [7,8]. Its expression in various cancers is usually associated with the prognosis. Thymidine phosphorylase (TP), survivin, and excision repair cross-complementing group 1 (ERCC1) and cyclin D1 have also been reported as predictive factors in anti-cancer therapy in GI cancer treated with 5-fluorouracil (5-FU) or cisplatin. These two agents are also commonly used in treatment of BDC; however, few data have been associated with these markers, except for TP in cholangiocarcinoma-derived cell lines [9].
In the current study, we investigated the clinico-pathological characteristics and expression of p53, TP, survivin, ERCC1, and cyclin D1 in order to compare the different characteristics among the three types of cancer.
Formalin-fixed, paraffin-embedded blocks from the surgically resected BDC tissues were studied. All of the samples were collected under protocols approved by the local Institutional Review Board (IRB). All patients were diagnosed with ICC, HC, or EHC at Seoul St. Mary's Hospital between January 1994 and May 2004. Of the total 104 patients, we obtained adequate tissue for immunohistochemical staining from 87 patients. We analyzed and compared the clinicopathological characteristics among the three types of cancer. A summary of the patients' general characteristics is shown in Table 1. All patients were followed up for at least five years.
For construction of the tissue microarray block, small core biopsies were taken from the non-necrotic, morphologically representative areas of the paraffin-embedded tumor tissues and were assembled on a recipient paraffin block. This was performed using a precision instrument (Micro Digital Co., Seoul, Korea). The biopsied core was 3.0 mm in diameter, which was sufficient for assessing the morphological features in the tissues, and 30 cores were then assembled on a recipient paraffin block. After construction, 5 µm sections were cut and hematoxylin and eosin staining was performed on the initial slide in order to verify the histology.
Immunohistochemical staining was performed on the 5 µm sections of the tissue microarray blocks. Paraffin sections were mounted on superfrost glass slides, deparaffinized, and rehydrated in a graded series of ethanol; they were then subjected to microwave antigen retrieval. Endogenous peroxides activity was blocked using 0.3% hydrogen peroxide. The sections were incubated for l hour at room temperature or at 4℃ overnight with the following primary antibodies at the specified dilutions: p53 (1:100, Dako Corp., Carpinteria, CA),TP (1:100, Zymed Laboratories, South San Francisco, CA), ERCC1 (1:200, NeoMarkers, Fremont, CA), survivin Ab-6 (1:50, NeoMarkers), and cyclin D1 (1:50, Dako Corp.), respectively. Immunohistochemical staining was performed using the rabbit or mouse DAKO ChemMate EnVision system, and the Peroxidase/DAB Kit (Dako). The sections were then counterstained with Mayer hematoxylin, and then dehydrated, cleared, and mounted.
The results were interpreted by two independent pathologists who were blinded to the specific diagnosis and prognosis for each case. Tumor cells that showed distinct nuclear or cytoplasmic staining of more than 10% of the cells were considered positive.
The SPSS ver. 10.0 (SPSS Inc., Chicago, IL) was used for analysis of the data. The immunohistochemical profiles were compared with the clinico-pathologic parameters using the chi-square test and the Fisher's exact test. We also used the Kaplan-Meier and Cox regression method for assessment of the survival function.
The median age of patients was 61 years (range, 36 to 80 years), male was more common (69 males, 35 females), and EHC was the most common, with 46 cases (44.2%). Of the 104 patients with potentially resectable disease on imaging study, three patients could not undergo R0 complete resection due to poor performance status, organ dysfunction, or difficult location for resection. Among the 101 patients who underwent complete resection, 53 patients (51.04%) were treated with 5-FU and cisplatin combination chemotherapy. At the median 5-year follow-up, three patients died of other causes within six months after surgery and seven patients were lost to follow up. Recurrenence of cancer occurred in 61 patients (58.7%) and 33 patients had a disease-free state (Table 1).
Although a relatively small number of cases of IHC were enrolled, no statistical difference in the portion of patients who underwent curative surgery was observed among the three types of cancer (p=0.347, data not shown).
Of the 104 patients, 73 cases (70.2%) showed moderately differentiated adenocarcinoma. Lymphatic invasion and venous invasion were not common in most cases (31.7% and 11.5%, respectively), however, perineural invasion was observed in 66.3% of cases. Among the three types of BDC, perineural invasion was significantly less common in ICC, compared with the other types (23.5% vs. 85.4%, 65.2%, respectively; p<0.001) (Table 2).
Expression of TP and p53 was most common in 50 cases and 43 cases (48.1% and 41.3%, respectively). Survivin expression was the next most common in 34 cases (32.7%). ERCC1 and cyclin D1 expression were less common in 26 cases and 19 cases (25.0% and 18.3%, respectively). However, no significant difference in expression of these proteins was observed among the three types of BDC (Table 3).
We analyzed clinical outcomes for 101 patients who underwent curative surgery. The median overall survival (OS) was 21.8 months (95% confidence interval [CI], 12.3 to 26.4 months). ICC showed poorer OS than other types of BDC, although no statistical difference was observed among the three types of BDC (15.9 months for ICC, 20.8 months for HC, and 21.9 months for EHC; p=0.956). The median time to recurrence (TTR) was 14.1 months (95% CI, 7.8 to 20.4 months). There was also no significant difference among the three types of cancer, however, ICC showed shorter TTR, compared with the other types (6.6 months for ICC, 18.2 months for HC, and 13.5 months for EHC; p=0.487).
We analyzed the clinico-pathologic characteristics as prognostic factors for each type of cancer. Adjuvant therapy showed significantly better survival in all three types. In terms of prognostic factors associated with overall survival, lymph node involvement, lymphatic and venous invasion showed prognostic significance in ICC, however, differentiation and ERCC1 expression had prognostic significance in HC and EHC (Table 4, Fig. 1).
Three types of BDC have been reported as having different clinical manifestations and prognostic factors [10,11], although these cancers originated from the same bile duct. They showed clinical symptoms and course, as well as radiologic findings, and are applied with different staging systems. For example, ICC is usually detected as an asymptomatic liver mass by imaging studies, however, EHC presents with jaundice or cholangitis. Therefore, these cancers are often regarded as different disease entities. However, clinical trials with new agents have been designed, regardless of type in BDC, due to their low incidence, leading to different clinical outcome according tothe portion of types enrolled. In practice, due to the lack of data from well controlled clinical trials, the same treatment protocols have been used. In view of the clinical course or prognostic factors, many different data have been introduced because each study resulted in various conclusions according to type of BDC enrolled in those studies. Until now, we were not able to determine adequate treatment planning for patients, especially in an adjuvant setting, therefore, in the current study, we attempted to identify differences among the three types of BDC.
In our data, ICC showed several different clinicopathologic characteristics distinguishing it from HC and EHC. First, ICC showed less common perineural invasion than HC or EHC. Perineural invasion is generally regarded as a prognostic factor in cancer of the biliary tract [12]. Although in some studies, perineural invasion was also frequently found in ICC with similar aggressiveness to that of other biliary tract cancers, these studies were conducted only in ICC with a small sample size, not a comparative study [13-15]. In addition, one previous study reported data similar to ours, where ICC had a smaller incidence of perineural invasion due to the anatomical location [5]. Although it is not conclusive because all of these studies were conducted with a small number of cases, perineural invasion can be a potential distinguishing clinical feature of ICC from other types of BDC.
In analysis of survival outcome, ICC showed the tendency of worse overall survival, compared with other types of BDC, however, no significant difference was observed. In terms of TTR, ICC also showed a trend of shorter time, compared with other types. The relatively small number of ICCs is limitation of our study, therefore, comparative study with a large number of patients should be conducted in order to determine whether ICC has poor survival outcome or not.
Several clinicopathologic findings have been suggested as a prognostic marker in BDC. We analyzed these prognostic factors according to each type of cancer for comparison of specific characteristics. Patients treated with adjuvant chemotherapy had better OS in all three types of cancer. No randomized, phase III clinical trial for adjuvant treatment in BDC has yet been conducted, therefore, there is no standard regimen or indication for adjuvant therapy in BDC after curative resection. However, our data suggested that adjuvant treatment can improve the clinical outcome in resectable BDC. Recently, new agents, including gemcitabine, capecitabine, or oxaliplatin have been tried for treatment of advanced BDC. Conduct of clinical trials using these agents can be considered for determination of the efficacy of adjuvant therapy in BDC. As other authors have previously mentioned [16,17], lymph node involvement, lymphatic invasion, and venous invasion were related to poor prognosis in ICC, but not in the other two types of BDC. However, the degree of differentiation was associated with prognosis in HC and EHC, but not in ICC. These are second differentiated points between ICC and the other two types of BDC. These factors can be important prognostic factors when trying to set the treatment plan for adjuvant therapy. Based on our result, a different treatment plan should be considered for design of clinical trials with BDC.
p53 and cyclin D1 have been studied as prognostic factors in BDC, however, the results varied according to the type of cancer enrolled. No prognostic significance was found in EHC in Korean data [18], however, in a meta-analysis, Chinese researchers suggested that high expression of p53 may be a prognostic factor in EHC [19]. In another study, differences in expression of cell cycle regulatory protein, such as p53 or p21, were observed in a small number of patients [20]. In the current study, we found no difference or prognostic significance among three types of BDC.
ERCC1 expression has been studied as a predictive or prognostic marker in various human cancers. Many studies have demonstrated its correlation with resistance to cisplatin or oxaliplatin and with survival outcome in lung, colorectal, gastric, and ovarian cancer [21]. However, no study has focused on ERCC1 expression in BDC, even though platinum agents such as cisplatin or oxaliplatin are commonly used for treatment of advanced BDC. In the current study, we found that ERCC1 expression showed correlation with poor survival outcome in HC and EHC, not ICC. We treated with 5-FU and cisplatin combination chemotherapy as adjuvant therapy; however, we did not analyze the association between ERCC1 expression and the chemotherapeutic agent. Further investigation with a large sample size will be needed for determination of an adequate regimen.
TP or survivin expression is also known to be a potential prognostic or predictive marker for anti-cancer therapy in GI cancer. TP is especially associated with the metabolism of capecitabine, therefore, this protein has been widely studied as a predictive marker in GI cancer [22]. Only one report has suggested that TP expression in ICC may herald enhanced aggressiveness with a 38.8% positivity rate [23]. According to one report, which included a small number of patients, nuclear survivin expression can also predict a poor survival outcome for patients with cholangiocarcinoma [24]. In our study, no significance as a prognostic factor or difference was observed among the three types of BDC.
In the current study, we found several different clinicopathologic findings between ICC and the other two types of BDC, and HC and EHC had similar features. Lymph node status, lymphatic and venous invasion can be prognostic factors in ICC, and the histological grade, ERCC1 expression can be considered as prognostic factors in HC and EHC. Based on our data, the treatment plan in ICC should be separated from that of other types of BDC. In an adjuvant setting, systemic chemotherapy should be considered in ICC if there is lymphatic or vascular invasion in histologic findings. For HC and EHC, adjuvant therapy can be applied for poorly differentiated type, and ERCC1 expression can be a potential prognostic marker for planning adjuvant therapy.
The data are very limited because this is retrospective analysis with a small sample size. However, our result suggested a rationale for establishment of a different treatment or clinical trial plan foreffective therapy in BDC. Conduct of further large, prospective trials is warranted in order to define these data.
Among three types of BDC, EHC, and HC had similar clinico-pathological features; however, ICC had different characteristics. Absence of lymph node involvement, well differentiated histology, adjuvant therapy, absence of lymphatic and venous invasion, and negative expression of TP and ERCC1 can be potential prognostic markers in BDC. Conduct of large scale prospective studies will be needed in order to confirm our data.
References
1. Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006; 45:856–867. PMID: 17030071.

2. Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001; 92:2374–2383. PMID: 11745293.
3. Weber A, Landrock S, Schneider J, Stangl M, Neu B, Born P, et al. Long-term outcome and prognostic factors of patients with hilar cholangiocarcinoma. World J Gastroenterol. 2007; 13:1422–1426. PMID: 17457974.

4. Sano T, Shimada K, Sakamoto Y, Ojima H, Esaki M, Kosuge T. Prognosis of perihilar cholangiocarcinoma: hilar bile duct cancer versus intrahepatic cholangiocarcinoma involving the hepatic hilus. Ann Surg Oncol. 2008; 15:590–599. PMID: 18057991.

5. Aishima S, Kuroda Y, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007; 31:1059–1067. PMID: 17592273.

6. Hong SM, Hwang I, Song DE, Choi J, Yu E. Clinical and prognostic significances of nuclear and cytoplasmic KIT expressions in extrahepatic bile duct carcinomas. Mod Pathol. 2007; 20:562–569. PMID: 17396144.

7. Arora DS, Ramsdale J, Lodge JP, Wyatt JI. p53 but not bcl-2 is expressed by most cholangiocarcinomas: a study of 28 cases. Histopathology. 1999; 34:497–501. PMID: 10383693.

8. Rashid A, Ueki T, Gao YT, Houlihan PS, Wallace C, Wang BS, et al. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002; 8:3156–3163. PMID: 12374683.
9. Thanasai J, Limpaiboon T, Jearanaikoon P, Sripa B, Pairojkul C, Tantimavanich S, et al. Effects of thymidine phosphorylase on tumor aggressiveness and 5-fluorouracil sensitivity in cholangiocarcinoma. World J Gastroenterol. 2010; 16:1631–1638. PMID: 20355241.

10. Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996; 224:463–473. PMID: 8857851.
11. Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol. 2009; 15:4240–4262. PMID: 19750567.

12. Cereda S, Belli C, Reni M. Adjuvant treatment in biliary tract cancer: to treat or not to treat? World J Gastroenterol. 2012; 18:2591–2596. PMID: 22690066.

13. Shirai K, Ebata T, Oda K, Nishio H, Nagasaka T, Nimura Y, et al. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2008; 32:2395–2402. PMID: 18795245.

14. Suzuki S, Sakaguchi T, Yokoi Y, Okamoto K, Kurachi K, Tsuchiya Y, et al. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg. 2002; 26:687–693. PMID: 12053220.

15. Tajima Y, Kuroki T, Fukuda K, Tsuneoka N, Furui J, Kanematsu T. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg. 2004; 91:99–104. PMID: 14716802.

16. Guedj N, Zhan Q, Perigny M, Rautou PE, Degos F, Belghiti J, et al. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol. 2009; 51:93–101. PMID: 19446907.

17. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011; 29:3140–3145. PMID: 21730269.

18. Kim WB, Han HJ, Lee HJ, Park SS, Song TJ, Kim HK, et al. Expression and clinical significance of cell cycle regulatory proteins in gallbladder and extrahepatic bile duct cancer. Ann Surg Oncol. 2009; 16:23–34. PMID: 18979138.

19. Wang J, Wang X, Xie S, Yan Z, Li Z, Li Y, et al. p53 status and its prognostic role in extrahepatic bile duct cancer: a meta-analysis of published studies. Dig Dis Sci. 2011; 56:655–662. PMID: 20668938.

20. Jarnagin WR, Klimstra DS, Hezel M, Gonen M, Fong Y, Roggin K, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol. 2006; 24:1152–1160. PMID: 16505435.

21. Gossage L, Madhusudan S. Current status of excision repair cross complementing-group 1 (ERCC1) in cancer. Cancer Treat Rev. 2007; 33:565–577. PMID: 17707593.

22. Toi M, Atiqur Rahman M, Bando H, Chow LW. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 2005; 6:158–166. PMID: 15737832.

23. Aishima S, Taguchi K, Sugimachi K, Asayama Y, Nishi H, Shimada M, et al. The role of thymidine phosphorylase and thrombospondin-1 in angiogenesis and progression of intrahepatic cholangiocarcinoma. Int J Surg Pathol. 2002; 10:47–56. PMID: 11927969.

24. Javle MM, Tan D, Yu J, LeVea CM, Li F, Kuvshinoff BW, et al. Nuclear survivin expression predicts poor outcome in cholangiocarcinoma. Hepatogastroenterology. 2004; 51:1653–1657. PMID: 15532797.
Fig. 1
Overall survival according to excision repair cross-complementing group 1 (ERCC1) expression. Intrahepatic cholangiocarcinoma (A) did not show any difference in overall survival, however, hilar cholangiocarcinoma (B) and extrahepatic cholangiocarcinoma (C) showed better survival outcome in ERCC 1 negative.
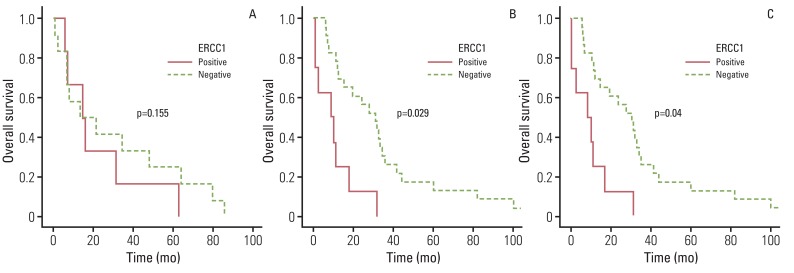
Table 1
Characteristics of patients
| Characteristic | No. (%) |
|---|---|
| Median age (range, yr) | 61 (36-80) |
| Gender (male/female) | 69/35 |
| Diagnosis | |
| ICC | 17 (16.3) |
| HC | 41 (39.4) |
| EHC | 46 (44.2) |
| Lymph node | |
| N0 | 67 (64.4) |
| N1 | 37 (35.6) |
| Stage | |
| I | 38 (36.5) |
| II | 46 (44.2) |
| III | 20 (19.2) |
| Adjuvant treatmenta) | |
| Yes | 53 (51.0) |
| No | 48 (46.2) |
| Recurrence | |
| Yes | 61 (58.7) |
| No | 33 (31.7) |
| Not availableb) | 7 (7.6) |
Table 2
The presence of invasion into adjacent structures in bile duct cancer
| Lymphatic invasion | Venous invasion | Perineural invasion | |
|---|---|---|---|
| ICC | 4 (23.5) | 3 (17.6) | 4 (23.5) |
| HC | 14 (34.1) | 5 (12.2) | 35 (85.4) |
| EHC | 15 (32.6) | 4 (8.7) | 30 (65.2) |
| p-value | 0.721 | 0.606 | <0.001 |
Table 3
Expression of p53, TP, ERCC1, survivin, and cyclin D1 in three types of bile duct cancer
Table 4
Comparison of prognostic factors among the three types of BDC




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download