Introduction
Materials and Methods
1. Study design
2. Chemotherapy
3. Efficacy and toxicity
4. Statistical consideration
Results
1. Patients' characteristics
Table 1
Table 2
| No. |
Age (yr) |
FIGO stage |
Histology | Prior chemotherapy |
Treatment time for FOLFOX-4 (mo) |
Cycles of FOLFOX-4 |
Tumor responsea) |
|---|---|---|---|---|---|---|---|
| 1 | 69 | IV | Serous |
T/C×9 → T/C×9 → D/C×7 → WT×5 To/P×3 → G/C×4 → WI×6 |
8 | 5 |
Partial response |
| 2 | 62 | IIIC | Serous | T/C×6 → To×6 → G/P×6 → PLD×4 → D/C×6 | 6 | 2 |
Progressive disease |
| 3 | 48 | IIIC | Serous | T/C×6 → D/P×6 → To/P×6 → G×4 | 5 | 2 |
Progressive disease |
| 4 | 65 | IIIC | Serous | T/C×9 → To/P×9 → G×1 | 4 | 10 |
Partial response |
| 5 | 46 | IIIC | Serous | T/C×9 → G/C×9 → D/P×3 → To×4 | 5 | 3 |
Progressive disease |
| 6 | 48 | IV | Mucinous | T/C×9 → G/C×2 | 3 | 3 |
Progressive disease |
| 7 | 63 | IIIC | Serous | T/C×9 → T/C×6 → G/C×3 → To×2 | 5 | 4 |
Partial response |
| 8 | 57 | IIIC | Serous | T/C×9 → G/C×4 → To×3 | 4 | 4 |
Stable disease |
| 9 | 53 | IIIC | Undifferentiated | T/C×9 → G/P×6 → To×3 | 4 | 10 |
Stable disease |
| 10 | 69 | IIB | Serous | T/C×6 → T/C×6 → G/P×6 → D×6→ To/P×9 | 6 | 1 |
Progressive disease |
| 11 | 60 | IIIA | Endometrioid | T/C×6 → G/P×6 | 3 | 7 |
Stable disease |
| 12 | 63 | IIIC | Serous |
T/C×6 → D/C×6 → G/C×6 → To×6 → E×6 → To/P×6 → WT×17 |
8 | 7 |
Stable disease |
| 13 | 78 | IIIC | Mucinous | T/C×9 → G/P×4 | 3 | 1 |
Progressive disease |
| 14 | 47 | IV | Serous |
T/C×3 → D/C×6 → G×4 → To×3 → G/P×3 → PLD×6 → Cy/P×6 → WT×3 |
9 | 2 |
Progressive disease |
| 15 | 56 | IV | Serous | T/C×7 → G/P×7 → To×3 | 4 | 7 |
Partial response |
| 16 | 64 | IIIC | Serous | T/C×9 → G/P×6 → To/P×3 → To/C×9 | 5 | 2 |
Progressive disease |
| 17 | 69 | IV | Serous | T/C×9 → To/P×6 → D/C×4 → G/C×2 | 5 | 4 |
Progressive disease |
| 18 | 50 | IIIC | Serous |
T/C×6 → T×6 → D/P×3 → To×2 → G/C×6 → To×4 → Cy/Do/P×9 → WI×1 |
9 | 4 |
Progressive disease |
| 19 | 60 | IIIC | Endometrioid | T/C×3 → To/P×3 → Cy/P×1 → G/P×6 → D×1 | 6 | 9 |
Partial response |
| 20 | 67 | IIIC | Serous |
T/C×9 → To×6 → G/C×6 → To×12 → D×2 → WI×2 → WT×5 |
8 | 7 |
Stable disease |
| 21 | 54 | IIIC | Endometrioid | T/C×7 → Do/P×4 → G/P×1 | 4 | 3 |
Progressive disease |
| 22 | 60 | IIIC | Endometrioid |
T/C×6 → G/C×6→ To/P×6 → WT×3 → Cy/P×6 → D×2 |
7 | 7 |
Stable disease |
| 23 | 59 | IIIC | Serous | T/C×9 → D/P×6 → To/P×6 → G×3 | 5 | 5 |
Progressive disease |
| 24 | 69 | IIIC | Serous | T/C×9 → To/P×9 | 3 | 2 |
Progressive disease |
| 25 | 79 | IIC | Serous | T/C×6 → D/C×9 → To×3 → G/P×4 | 5 | 2 |
Progressive disease |
| 26 | 50 | IIIB | Serous | T/C×9 → To/P×6 → D/C×4 | 4 | 7 |
Partial response |
| 27 | 70 | IIIC | Serous | T/C×9 → D/C×3 → To×9 → To/P×6 | 5 | 6 |
Partial response |
| 28 | 60 | IIIC | Serous | T/C×9 → To/P×3 → G/C×3 | 4 | 2 |
Progressive disease |
FOLFOX-4, oxaliplatin, leucovorin, and 5-fluorouracil; FIGO, International Federation of Gynecology and Obstetrics criteria for ovarian cancer; T, paclitaxel; C, carboplatin; D, docetaxel; WT, weekly paclitaxel; To, topotecan; P, cisplatin; G, gemcitabine; WI, weekly irinotecan; PLO, pegylated liposomal doxorubicin; E, etoposide; Cy, cyclophosphamide; Do, doxorubicin. a)Tumor response according to the Response Evaluation Criteria in Solid Tumors.
Table 3
Values are presented as number (%). FOLFOX-4, oxaliplatin, leucovorin, and 5-fluorouracil; FIGO, International Federation of Gynecology and Obstetrics; WHO, World Health Organization; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; TTPD, time to progressive disease; CSS, chemotherapy-specific survival.
2. Efficacy
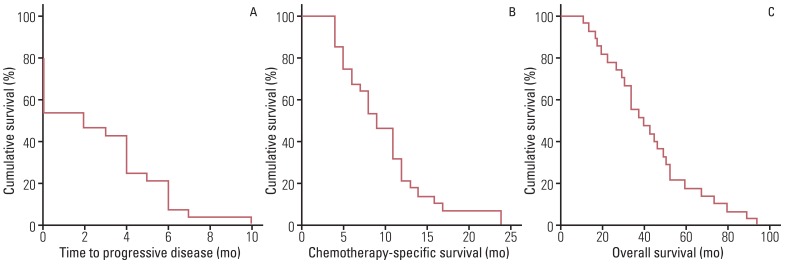 | Fig. 1Kaplan-Meier survival analysis with the log-rank test for (A) time to progressive disease, (B) chemotherapy-specific survival, and (C) overall survival in 28 patients with recurrent epithelial ovarian cancer who received oxaliplatin, leucovorin, and 5-fluoleurouracil (FOLFOX-4) chemotherapy. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download