Abstract
Tumor lysis syndrome (TLS) has rarely been observed in solid tumors. We report on a case of a patient with advanced invasive thymoma who developed tumor lysis syndrome after chemotherapy. The potential complications of TLS should be considered in treatment of extensive thymoma.
Tumor lysis syndrome (TLS) is an oncologic emergency characterized by hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, and is caused by destruction of a large number of rapidly proliferating neoplastic cells. Development of acidosis may also occur, and acute renal failure requiring hemodialysis is a frequent occurrence. Although TLS may occur spontaneously before administration of therapy, it is most commonly observed after initiation of cytotoxic chemotherapy, primarily in hematologic malignancies with a high proliferative rate such as that of Burkitt's lymphoma and acute lymphoblastic leukemia. However, its occurrence in association with solid cancers is relatively uncommon, and it has rarely been observed in patients with thymoma. Here we report on a patient with stage IVa, World Health Organization (WHO) type B3 thymoma who was treated for tumor lysis syndrome after initiation of third-line chemotherapy.
A 40-year-old female presented to the emergency room with a three-day history of progressive dyspnea due to pleural effusion in February 2010. A computed tomography (CT) scan of the chest showed an extensive soft-tissue mass, measuring 12×8 cm in size, in the anterior mediastinum extending into the right hemithorax and pericardial space (Fig. 1A and B). Percutaneous gun biopsy of the right anterior mediastinal mass confirmed WHO type B3 thymoma (Fig. 2), classified according to the Masaoka clinical staging system. Due to the unresectable state of disease, neoadjuvant chemotherapy was scheduled in order to reduce the tumor size prior to surgery. From February 19 to April 5, 2010, the patient received three cycles of systemic chemotherapy with a regimen consisting of docetaxel (75 mg/m2 i.v. over 1 hour on day 1) and cisplatin (75 mg/m2 i.v. over 1 hour on day 1). Despite a slight interval decrease in the size of thymoma and a decreased amount of pericardial and pleural effusion after three cycles of chemotherapy, two more cycles of chemotherapy were administered due to incomplete resectability and patient's reluctance.
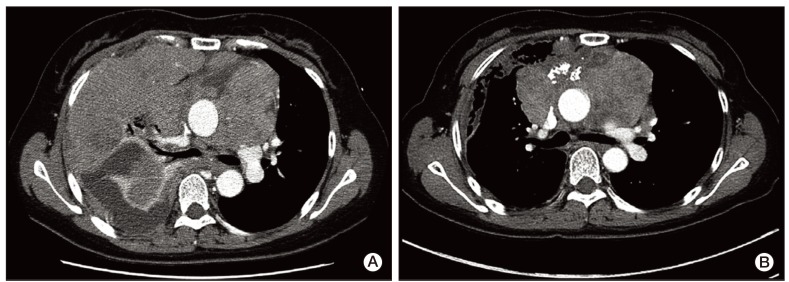
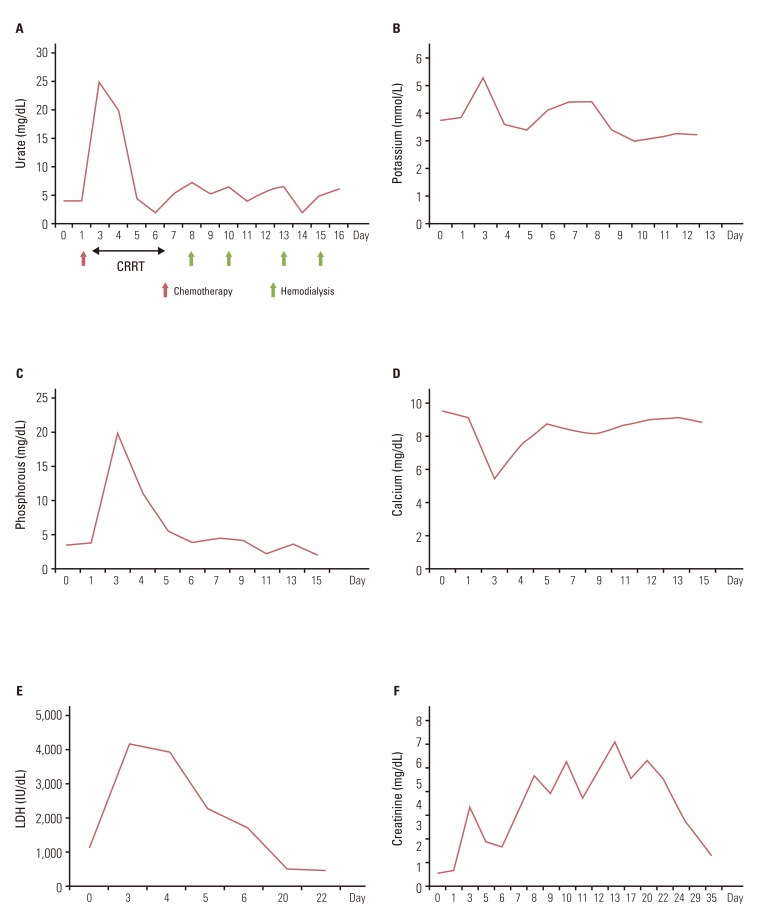
Due to disease progression during follow-up, the patient received four additional cycles of systemic chemotherapy with a regimen consisting of cyclophosphamide (500 mg/m2 i.v. over 1 hour on day 1), doxorubicin (50 mg/m2 i.v. over 30 minutes on day 1), and cisplatin (50 mg/m2 i.v. over 1 hour on day 1) from May 11 to July 26, 2010. The disease status remained stable for 11 months after second-line chemotherapy, and thereafter showed slow progress. On April 12, 2012, the patient complained of aggravation of dyspnea and chest X-ray showed massive pleural effusion on the right side of the chest. Chest CT showed aggravation of anterior mediastinal thymoma with extension into the middle mediastinum and surrounding pericardium as well as the entire right side of pleura (Fig. 3A). A percutaneous pig-tail catheter was inserted into the right pleural space for drainage of malignant pleural effusion. Third-line chemotherapy was started on April 18, 2012, with a regimen consisting of paclitaxel (175 mg/m2 i.v. over 3 hours on day 1), ifosfamide (2,500 mg/m2 i.v. over 2 hours on days 1 and 2), and mesna (500 mg/m2 i.v. over 15 minutes on days 1 and 2). After completion of one cycle of chemotherapy, tachypnea, tachycardia, and oligouria were detected on the second day of chemotherapy. Blood chemistry showed the following: potassium, 5.3 mmol/L (3.5-5.1 mmol/L); phosphorus, 20.0 mg/dL (2.5-4.5 mg/dL); uric acid, 25.0 mg/dL (2.4-6.0 mg/dL); calcium, 5.5 mg/dL (8.4-10.2 mg/dL); arterial blood gas analysis, pH 7.24 (7.35-7.45); bicarbonate, 9.8 mmol/L (21.0-31.0 mmol/L); urea nitrogen, 73.6 mg/dL (8.0-22.0 mg/dL); creatinine, 3.74 mg/dL (0.6-1.1 mg/dL); lactate dehydrogenase (LDH), 4,153 IU/L (240-480 IU/L); and urine pH, 5.5 (5.0-8.0). Based on the patient's clinical symptoms and consequent laboratory findings, it was determined that the patient had developed classical signs of TLS, including acute kidney injury. The patient was immediately transferred to the intensive care unit for treatment, including aggressive hydration, intravenous bicarbonate for urine alkalinization, allopurinol, and repeated hemodialysis. On day 3, continuous renal replacement therapy was started and continued until day 5. During the following days, the patient's clinical symptoms and signs, such as dyspnea, tachypnea, and tachycardia, showed improvement, and the biochemical markers, including metabolic acidosis, hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, returned to normal (Fig. 4A-D). The plasma level of LDH was 1,139 IU/L before chemotherapy, increasing to 4,153 IU/L on day 3, and decreased to 498 IU/L on day 22 (Fig. 4E).
Hemodialysis was performed twice per week from April 25 to May 9, due to persistent azotemia despite adequate urine output (Fig. 4F). Serum urea nitrogen and creatinine concentration decreased to 15.2 mg/dL and 1.45 mg/dL, respectively, on day 35; therefore, further hemodialysis was not required. Of particular interest, remarkable regression of the preexisting tumor after one cycle of third-line chemotherapy was observed on a follow-up chest CT scan (Fig. 3B). The patient has remained on the same regimen with partial response to chemotherapy without recurrence of TLS.
Most cases of TLS occur in hematological malignancies, acute leukemia, or lymphoma [1,2]. Early recognition and subsequent prevention of TLS in patients who are susceptible to its development are essential in management of hematological tumors. Consequently, tests for serum electrolytes, uric acid, phosphorus, and renal function are monitored closely during treatment of these malignancies. However, TLS is rarely observed in solid tumors and is limited to individual case reports [3]. A literature review identified only 45 cases from its first report from 1977 to 2002 [4]. Among these cases, 37 cases occurred in tumors of highly sensitive tumor types (small cell lung cancer, germ cell tumor, neuroblastoma, and medulloblastoma) or moderately sensitive tumor types (breast cancer, non-small-cell lung cancer, ovarian cancer, colorectal cancer, and thymoma). However, eight cases occurred in tumor types (melanoma, sarcoma, and hepatocellular carcinoma) traditionally regarded as being relatively insensitive to therapy. In six of these latter cases, the patients presented with bulky, metastatic disease, which was also seen in our case [4]. It is possible that the improvement of systemic treatment for solid tumors accompanied by increasing responses seen in recent years may potentially lead to increased incidence of TLS. Risk factors for TLS in patients with solid tumors are high tumor volume with metastatic presentation, liver metastasis, and the classical risk factors such as high LDH level, high uric acid level, and pre-existent renal insufficiency [5].
Nevertheless, TLS in a patient with a thymoma is extremely rare. In addition, thymoma is considered a chemotherapy-sensitive disease. In this case, the patient had normal renal function prior to treatment, but had significantly increased levels of LDH post-treatment. She also had bulky disease with dissemination of pleura and pericardium. These manifestations appear to have been associated with predisposing factors of TLS. With optimal management of TLS, the patient recovered from clinical and biochemical derangement. However, it is of note that development of TLS did not occur after two lines of previous chemotherapy, including docetaxel plus cisplatin or cyclophosphamide, adriamycin, and cisplatin, which are similar to paclitaxel plus ifosfamide. Thus, it is not clear why the patient developed TLS only after a third line of chemotherapy despite similar extent of disease status.
Although re-biopsy at the time of progression was not performed in this case, there is a possibility that the primary tumor became sensitive to chemotherapy over time, suggesting tumor heterogeneity.
For prevention of TLS, all patients, including those with solid tumors who are at high risk for TLS should receive intravenous hyperhydration in order to achieve rapid improvement of renal perfusion and glomerular filtration and to minimize acidosis (which lowers urine pH and promotes precipitation of uric acid crystals) and oliguria [4]. The uric acid level should be reduced with use of allopurinol, particularly with use of rasburicase. However, the use of alkalization is controversial. In theory, production of alkaline urine will improve excretion of uric acid; however, vigorous urinary alkalization may increase the likelihood of calcium phosphate precipitation in renal tubules. Therefore, bicarbonate should be administered with caution. Management should also include prevention of cardiac dysarrhythmias and neuromuscular irritability. Despite optimal care, development of severe acute kidney injury may occur in some patients and requires renal replacement therapy [4,6].
In general, the incidence of TLS in solid tumors is too rare to justify administration of preventive therapies in all patients. In addition, TLS in solid tumors typically occurs within a few days to weeks after treatment, usually after the patient has been discharged [4]. This may be related to slow and delayed response to antineoplastic treatments or lysis of tumor cells at different time intervals due to different cell cycles in solid tumor cells, in contrast to synchronous cell death in haematological malignancies [5]. This may explain why the mortality associated with TLS in solid tumors is higher than that in hematological cancers [5,7,8].
This case highlights the importance of evaluation of potential risk factors of TSL and preventive therapies needed even for treatment of solid tumors. Greater caution should be exercised in patients with extensive metastatic disease who are sensitive to chemotherapy with known pretreatment risk factors, including renal impairment, hyperuricemia, and increased LDH.
References
1. Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010; 55(5 Suppl 3):S1–S13. PMID: 20420966.

2. Cairo MS, Coiffier B, Reiter A, Younes A. TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010; 149:578–586. PMID: 20331465.

3. Mott FE, Esana A, Chakmakjian C, Herrington JD. Tumor lysis syndrome in solid tumors. Support Cancer Ther. 2005; 2:188–191. PMID: 18628171.

4. Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors: a case report and review of the literature. Cancer Chemother Pharmacol. 2003; 51:187–192. PMID: 12655435.
5. Gemici C. Tumour lysis syndrome in solid tumours. Clin Oncol (R Coll Radiol). 2006; 18:773–780. PMID: 17168213.

6. Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011; 364:1844–1854. PMID: 21561350.

7. Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies: report of a case and review of the literature. Am J Clin Oncol. 1994; 17:502–505. PMID: 7977169.
8. Castro MP, VanAuken J, Spencer-Cisek P, Legha S, Sponzo RW. Acute tumor lysis syndrome associated with concurrent biochemotherapy of metastatic melanoma: a case report and review of the literature. Cancer. 1999; 85:1055–1059. PMID: 10091788.
Fig. 1
(A) Chest X-ray, obtained at diagnosis. Huge anterior mediastinal mass extending into the right lung with right pleural effusion. (B) Contrast-enhanced computed tomographic scan, obtained at diagnosis. Extensive soft-tissue mass in the anterior mediastinum extending into the right hemithorax and pericardial involvement.
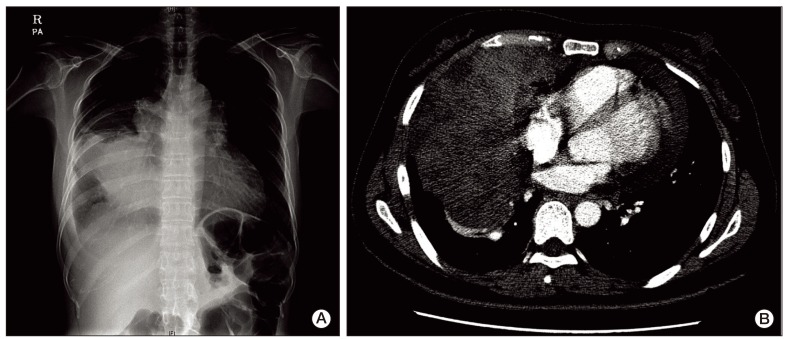
Fig. 2
Biopsy specimen of the tumor. The tumor was characterized by a predominant population of thymic epithelial cells and a significantly reduced small lymphocytic population (H&E staining, ×300).
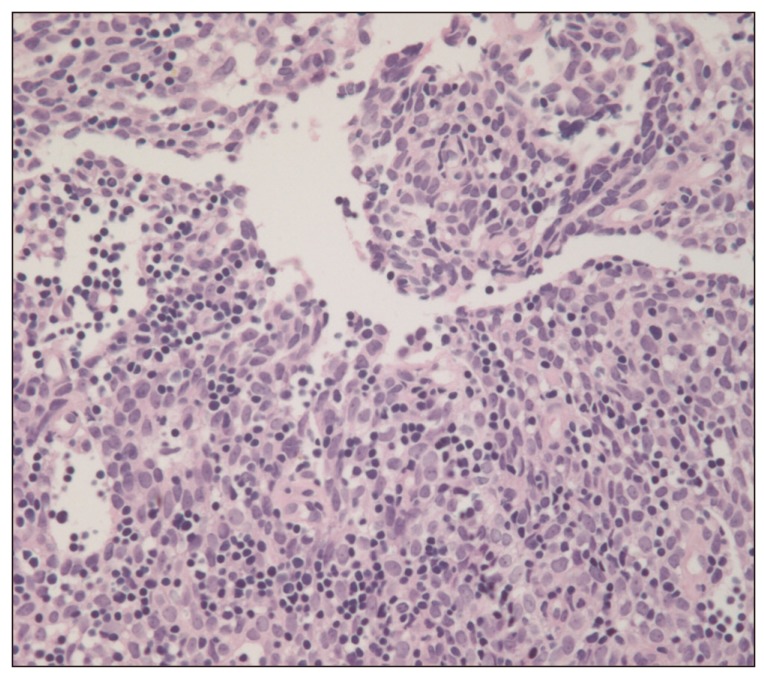
Fig. 3
(A) Contrast-enhanced computed tomographic scan, obtained at progression showed increased size of thymoma, causing narrowing of the superior vena cava and compression of the carina. (B) Contrast-enhanced computed tomographic scan, obtained after two cycles of paclitaxel and ifosfamide combination chemotherapy showed marked interval improvement of the anterior mediastinal mass with pleural and pericarial seeding.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download