Abstract
Purpose
Although influenza is regarded as a major cause of morbidity and mortality in immunocompromised patients, vaccine coverage remains poor. We evaluated the immunogenicity of influenza vaccines in colorectal cancer patients.
Materials and Methods
In this study, 40 colorectal cancer patients who received an influenza vaccine at the Korea Cancer Center Hospital during the 2009-2010 and 2010-2011 influenza seasons were analyzed. The blood samples were collected at prevaccination and 30 days post vaccination, and antibody titers were measured using the hemagglutination-inhibition tests.
Results
In the 2009-2011 season, the seroprotection rate for H1N1 (94.7%) was significantly higher than that for H3N2 (42.1%) and B (47.3%). The seroconversion rate was 52.6%, 26.3%, and 36.8% for H1N1, H3N2, and B, respectively. Fold increase of geometric mean titer (MFI) was 3.86, 1.49, and 3.33 for H1N1, H3N2, and B, respectively. In the 2010-2011 season, the seroprotection rate for H1N1 (57.1%) was significantly higher than that for H3N2 (52.4%) and B (38.1%). The seroconversion rate was 52.4%, 47.6% and 33.3% for H1N1, H3N2, and B, respectively. MFI was 12.29, 3.62 and 4.27 for H1N1, H3N2, and B, respectively.
Go to : 
Influenza is a common cause of respiratory tract infection during an epidemic season. Influenza has a significant clinical impact in immunocompromised patients, including adults with cancer, and can cause severe complications. In particular, patients after hematopoietic stem cell transplantation and those undergoing chemotherapy for leukemia are at highest risk, with high morbidity from complications, such as pneumonia, and a clinically relevant mortality rate [1-3]. Influenza represents a significant socio-economic burden on individuals and the community. Although the immunogenicity of influenza vaccine varies annually, it is well accepted that an influenza vaccine can reduce morbidity and disease severity. Despite the vaccine having weaker immunogenicity in immunocompromised patients than in healthy subjects, it is still recommended for use in such patients for the prevention of severe influenza or superimposed bacterial infections [4,5].
In Korea, administration of an influenza vaccine to immunocompromised patients, including adult cancerpatients, is recommended prior to the winter seasons. However, no mass medical survey of vaccine coverage in immunocompromised patients has been conducted, and it is presumed that the actual vaccine coverage remains poor in high-risk patients, as is the case in other countries [6-9]. Loulergue et al. [10] reported that the main reasons for low vaccine coverage included lack of promotion by the treating physician (72%), fear of side-effects (33%), and concerns regarding the vaccination efficacy (10%). Among medical oncologists, the leading self-reported reason for the lack of vaccination was due to minimal awareness of recommendations [10]. There was also a concern regarding the efficacy of influenzavaccination in immunocompromised patients [10].
Colorectal cancer is estimated as the third most frequent cancer (second in male and third in female) in Korea according to 2012's report [11]. Due to a currently rapid growing incidence, the importance about colorectal cancer is also growing in Korea; also because, different from other cancers, the chemotherapy regimen for colorectal cancer was well established, it is easy to identify the influences of chemotherapy on the immunogenicity of influenza vaccine. Further, because colorectal cancer develops more frequently in older age, both aging and immune-compromising factors are supposed to act as the reducing factors in the immunogenicity of influenza. This study aims to answer the question: If older colorectal patients were vaccinated, what the immunogenicity will be like?
The primary aim of this study is to evaluate the influenza vaccine immunogenicity in a group of high-risk subjects, colorectal cancer patients, from the Korea Cancer Center Hospital (KCCH), which is a single institute experience, and to offer a reference for immunization guidelines. The secondary aim of this study is to investigate the factors (immune status, duration of chemotherapy, chemo regimen, etc.) influencing the immunogenicity of influenza vaccine in colorectal patients.
Go to : 
During the 2009-2010 and 2010-2011 influenza seasons, patients with colorectal cancer, under treatment at KCCH, were invited to participate in this study. Institutional review board's approval was obtained, and the subjects gave written informed consent for blood collection for hemagglutination inhibition (HI) assays and 1-2 months post-vaccination to assess the immune response to influenza vaccination. The baseline patient demographics, including age, sex, tumor stage, surgery history, and type of chemotherapy, were collected. Subjects with severe allergy to an influenza vaccine or egg protein, with acute febrile illness at the time of vaccination, who were receiving treatment with corticosteroids for other reasons except for the purpose of anticancer drug with a history of transfusion within 6 months, or with any other condition that might interfere with the evaluation of the study were excluded. Paired blood samples were obtained for immunogenicity analysis from all subjects. On day 0 and days 30-60 after the first vaccination, 5-mL venous blood samples were obtained from all subjects. Subjects were excluded from an immunogenicity analysis if they were found to be non-compliant with the immunization or blood sampling schedule.
SK influenza IX vaccine (trivalent split influenza vaccine; SK Chemicals, Seongnam, Korea) was administered intramuscularly to all subjects during the 2009-2010 and 2010-2011 influenza seasons. In the 2009-2010 influenza season, SK influenza IX vaccinecontained 15 µg of each hemagglutinin from A/Brisbane/59/2007 (H1N1) strain (IVR-148), A/Uruguay/716/2007 (H3N2) strain (NYMCX-175C), and B/Brisbane/60/2008 strain. In the 2010-2011 influenza season, SK influenza IX vaccine contained 15 µg of each hemagglutinin from A/California/7/2009 (reassortant NYMC X-181; pandemic H1N1, pH1N1) strain, A/Victoria/210/2009 (reassortant NYMC X-187; H3N2) strain, and B/Brisbane/60/2008 strain.
The HI test, using the chicken red blood cells, was performed to determine the anti-hemagglutinin antibody titers. Anti-hemagglutinin titers to H1N1, H3N2, and influenza B were measured using A/Brisbane/59/2007 IVR-148 (H1N1), A/Uruguay/716/2007 NYMCX-175C (H3N2), and B/Brisbane/60/2008 in the 2009-2010 season, and A/California/7/2009 (reassortant NYMC X-181; pH1N1), A/Victoria/210/2009 (reassortant NYMC X-187; H3N2), and B/Brisbane/60/2008 in the 2010-2011 season.
Seroprotection was defined as an anti-HI titer≥1:40. Seroconversion was defined as a change from the baseline titer < 1:10 to a post-vaccination titer≥1:40 or a 4-fold or greater rise in titer in those with the initial titer≥1:10. Immunogenicity of the vaccine was assessed based on these findings: seroprotection rates on days 0 and 30, seroconversion rate on day 30, and mean fold increase (MFI) of geometric mean titer (GMT) of the HI assay between days 0 and 30.
In order to confirm the protective immunogenicity of the vaccine based on the European Medicines Agency criteria, one of the following criteria needed to be met: seroprotection rate>70% for subjects aged 18-60 years and>60% for subjects aged>60 years, seroconversion rate>40% for subjects aged 18-60 years and>30% for subjects aged>60 years, or MFI>2.5 for subjects aged 18-60 years and>2.0 for subjects aged>60 years [12]. We defined an acceptable but limited immune response at the seroprotection rate of 30-70%.
SPSS ver. 12.0 (SPSS Inc., Chicago, IL) was used for all analyses. One-sample t-test, independent-sample t-test, one-way analysis of variance (ANOVA), Kruskal-Wallis test, and Levene's test were used for the assessment of vaccine immunogenicity. Multivariate logistic regression analysis was used for identifying various factors influencing on the immunogenicity of influenza vaccine for colorectal cancer patients.
Go to : 
A total of 62 patients with colorectal cancer at KCCH were enrolled in this study from October 2009 to December 2010. We excluded 22 patients because paired samples were not available, leaving 40 patients with colorectal cancer available for evaluation. The mean age was 61.1 years (range, 26.52 to 88.68 years; 66.0 years in the 2009-2010 season and 56.70 years in the 2010-2011 seasons). The male/female ratio was 26/14 (11/8 in the 2009-2010 seasons and 15/6 in the 2010-2011 seasons). Table 1 shows the characteristics of the patients. In total, 17 of 19 patients with colorectal cancer enrolled in 2009 were treated with surgery and chemotherapy, and the remaining 2 patients received only surgery. Twenty-one patients enrolled in 2010 received chemotherapy after surgery. All subjects received influenza vaccination when their absolute neutrophil count (ANCs) was over 1,000 during the bone marrow recovery phase after previous chemotherapy. The average white blood cell (WBC) count was 5,476/µL, and the average ANC was 3,627 on the day of influenza vaccination. The average interval between influenza vaccination and post-vaccination sampling was 33.2 days in the 2009-2010 season and 44.15 days in the 2010-2011 seasons (Table 1).
Characteristics of the subjects with colorectal cancer
Before vaccination, 68.4% (n=13), 31.6% (n=6), and 10.5% (n=2) of 19 patients enrolled in the 2009-2010 season had seroprotective antibody titers (≥1:40) against H1N1, H3N2, and B, respectively. After vaccination, 94.7% (n=18), 42.1% (n=8), and 47.3% (n=9) of patients had seroprotective titers against H1N1, H3N2, and B, respectively. Seroconversion was observed in 52.6% (n=10), 26.3% (n=5), and 36.8% (n=7) of patients against H1N1, H3N2, and B, respectively. GMT fold increase was 3.86, 1.49, and 3.33 against H1N1, H3N2, and B, respectively (Table 2). These seroprotection and seroconversion rates indicated good immunogenicity (seroprotection rate≥70%, seroconversion≥40%) against H1N1, and an acceptable but limited immune response against influenza H3N2 and B. The GMT fold increase indicated good immunogenicity (GMT fold increase≥2.5) against all vaccine strains in the 2009-2010 season.
Immunogenicity of influenza vaccine for colorectal patients in each influenza season
Before vaccination, 4.8% (n=1), 4.8% (n=1), and 4.8% (n=1) of 21 patients enrolled in the 2010-2011 season had seroprotective antibody titers (≥1:40) against pH1N1, H3N2, and influenza B, respectively. After vaccination, the seroprotection rates were 57.1% (n=12), 52.4% (n=11), and 38.1% (n=8), respectively, against these strains. Seroconversion was observed for 52.4% (n=11), 47.6% (n=10), and 33.3% (n=7) of patients against pH1N1, H3N2, and influenza B, respectively. GMT fold increase was 12.29, 3.62, and 4.27 against the respective strains (Table 2). This seroprotection rate indicated an acceptable but limited immune response against all vaccine strains in the 2010-2011 seasons. However, the seroconversion rate indicated a good immunogenicity (seroconversion≥ 40%) against pH1N1 and H3N2 strains, while there was an acceptable but limited immune response against the influenza B strain. The GMT fold increase indicated a good immunogenicity (GMT fold increase≥2.5) against all vaccine strains in the 2010-2011 seasons.
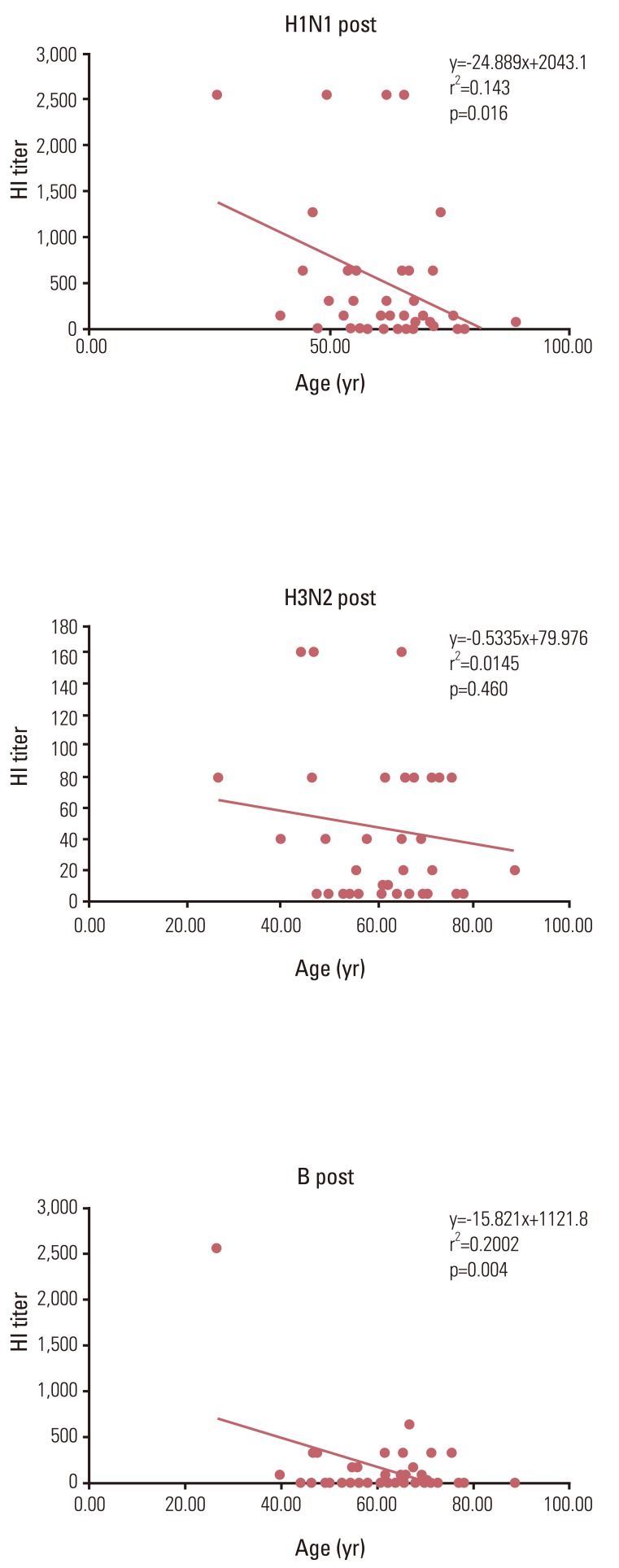
For an assessment of the effects of age on immunogenicity, linear regression analysis between the age of subjects and their HI titer was applied for all subjects. For each H1N1 and B, there was a significant reverse linear correlation between the age of subjects and their HI titer. (r2=0.143, p=0.016 and r2=0.20, p=0.004 for each H1N1 and B) (Fig. 1).
We applied a cut-off value of 65 years to subjects for further analysis, because it was well known that an individual of>65 year of age has poor immunogenicity to an influenza vaccine. For assessment, subjects were divided into 2 age groups (<65 years and≥65 years).
In the 2009-2010 season, the seroprotection rates against H1N1, H3N2, and influenza B were 100.0%, 28.6%, and 71.4%, respectively, in those aged <65 years; these values were 91.7%, 50.0%, and 33.3%, respectively, in those aged≥65 years. The seroconversion rates against H1N1, H3N2, and influenza B were 57.1%, 14.3%, and 57.1%, respectively, in those aged <65 years; these values were 50.0%, 33.3%, and 25.0%, respectively, in those aged≥65 years. GMT fold increases against H1N1, H3N2, and influenza B were, 5.38, 1.00, and 5.94, respectively, in those aged <65 years; these values were 6.56, 2.97, and 4.00, respectively, in those aged≥65 years. There were no statistically significant differences in the immune responses between the 2 age groups in the 2009-2010 seasons, except in post-vaccination GMT against H3N2 (Table 3).
Comparison of immunogenicity of influenza vaccine between colorectal cancer subjects aged<65 years and ≥65 years in 2009-2010 influenza season
In the 2010-2011 season, the seroprotection rates against pH1N1, H3N2, and influenza B were 60.0%, 46.7%, and 26.7%, respectively, for those aged <65 years; these values were 50.0%, 66.7%, and 66.7%, respectively, for those aged≥65 years. Seroconversion rates against pH1N1, H3N2, and influenza B were 53.3%, 40.0%, and 26.7%, respectively, in those aged <65 years; these values were 50.0%, 66.7%, and 50.0%, respectively, in those aged≥65 years. GMT fold increases against pH1N1, H3N2, and influenza B were 4.88, 2.00, and 1.81, respectively, in those aged <65 years; these values were 6.35, 7.13, and 7.13, respectively, in those aged≥65 years. For all strains, there were no statistically significant differences in the immune responses between the 2 age groups in the 2010-2011 seasons (Table 4).
Comparison of immunogenicity of influenza vaccine between colorectal cancer subjects aged <65 years and ≥65 years in 2009-2010 influenza season
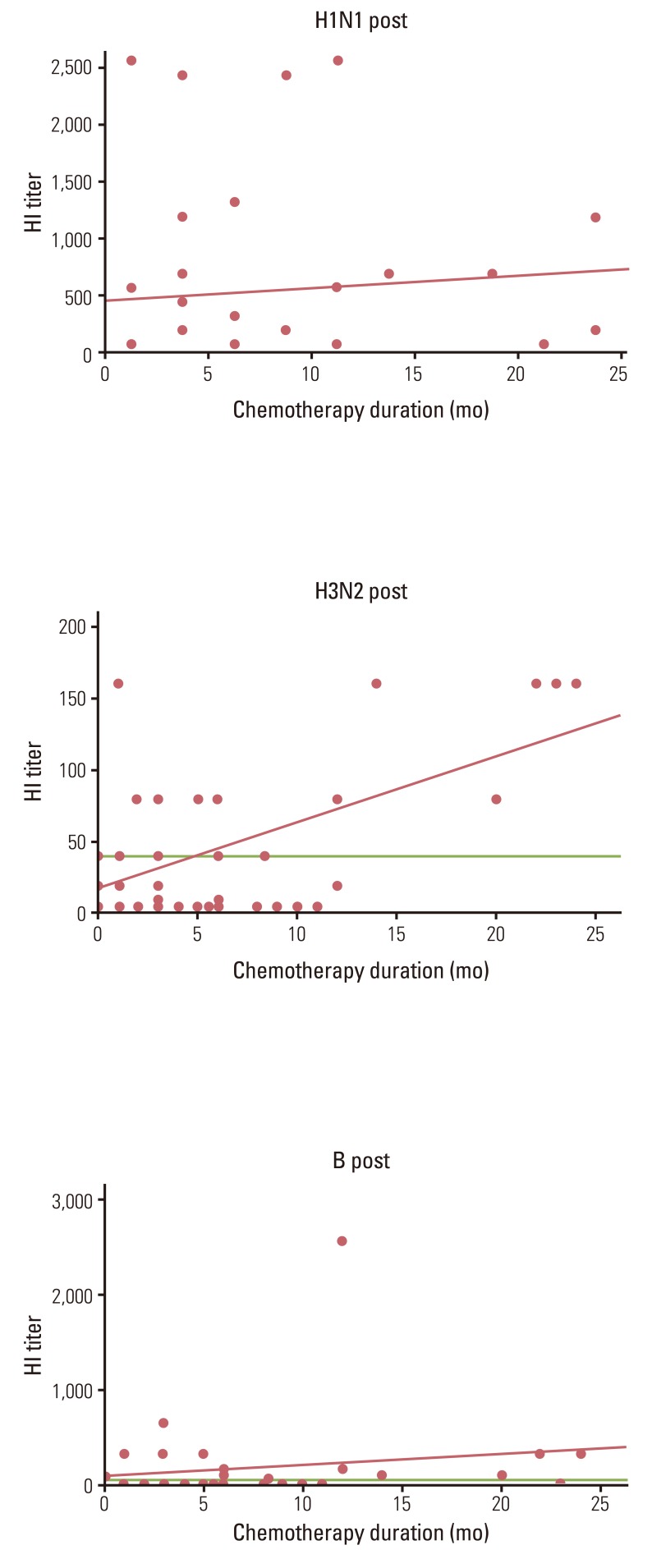
For an assessment regarding the effects of chemotherapy on the influenza vaccine immunogenicity, linear regression analysis between recent and total chemotherapy duration, chemotherapy types (adjuvant, palliative, adjuvant, and palliative combined) and their HI titer was applied for all subjects without the respect of different influenza seasons. For H3N2, there was a significant linear correlation between total chemotherapy duration and its HI titer (r2=0.313, p=0.000). However, there were no correlations between total chemotherapy duration and its HI titer for H1N1and B (Fig. 2). We applied a cut-off value for each 2, 4, 6, 8, 10, and 12 months of recent chemotherapy duration to subjects for further analysis. There were no differences in the seroprotection rates to influenza viruses between the two groups, according to the cut-off value.
For an assessment regarding the effects of immune status on the influenza vaccine immunogenicity, linear regression analysis between WBC count, ANC, absolute lymphocyte count (ALC) and their HI titer was applied for all subjects without the respect of different influenza seasons. For B, there was a significant linear correlation between ANC and its HI titer (r2=0.148, p=0.017). However, there were no correlations between ANC and its HI titer for H1N1and H3N2. There were no correlations between WNC count, ALC and its HI titer for all strains. When applied the cut-off value 1,000 for ANC and ALC, there were no differences of the immune response to influenza vaccine between subjects with≥1,000 and subjects with<1,000.
On further analyses, stage of colorectal cancer and recurrence state at vaccination did not affect the immune response to influenza antigens.
On day 30 post-vaccination, participants were asked to report any adverse effects of the vaccination. Reported side effects included some local adverse reactions; however, serious adverse effects, such as Guillain-Barre syndrome, were not reported. Overall, the vaccine was well tolerated in all subjects.
Go to : 
This study demonstrated the safety of the trivalent, an inactivated influenza vaccine in colorectal cancer patients, with an acceptable but limited immune response. This result matched well with the results of previous studies in immunocompromised patients, especially in colorectal cancer patients [13-16].
Puthillath et al. [13] reported that 70.6% of 85 colorectal cancer patients vaccinated with an influenza vaccine showed an immune response during the 2006-2007 influenza seasons.
In the present study, the seroprotection rate was 94.7% for H1N1, 42.1% for H3N2, and 47.4% for influenza B in the 2009-2010 seasons. The seroprotection rate against H1N1 was significantly higher than that against H3N2 and influenza B in the 2009-2010 seasons. The high seroprotection rate against H1N1 was probably a result of a high pre-seroprotection rate against that strain. The cause of the high pre-seroprotection rate against H1N1 might have been the long-term homologous H1N1 vaccine antigen stimuli. In the 2007-2008 influenza seasons, there was some mismatch between the circulating wild strain and the vaccine strain, and the vaccine strain was subsequently changed from A/Wisconsin/67/2005 to A/Brisbane/10/2007 (H3N2). It was supposed that a short-term H3N2 vaccine antigen stimulus was unable to induce a good immune response, especially in immunocompromised patients. Regarding the relatively poor immune response to B antigen, our results were similar to the results of the study performed by Xie et al. [17]. They suggested that the low immunogenicity of B/Brisbane/60/2008 was probably due to the fact that it was a new strain, unlike the A/Brisbane/59/2007 (H1N1) strain, which had been a vaccine component since the 2008-2009 season [17].
Compared with the control group (diabetes mellitus patients, n=15) vaccinated in the 2009-2010 season, there were no differences in the immunogenicity, except against the influenza B strain. In the control group, the pre-seroprotection rate and GMT were statistically significantly higher against influenza B. Therefore, direct comparisons between the study and control groups for the vaccine immunogenicity against influenza B strains were not possible (Table 5).
Comparison of immunogenicity of influenza vaccine between CRC (n=19) and DM (n=15) subjects in 2009-2010 influenza season
Age, type of malignancy, WBC count, lymphocyte count, serum immunoglobulin G (IgG) level, and status of cancer therapy are well known factors affecting the immune response after an influenza vaccination [18]. In this study, age and ANC showed a correlation with the immune response for some strains, but status of cancer therapy, WBC count, and lymphocyte count did not show a significant correlation with the immune response. Furthermore, in this study, total chemotherapy duration showed a correlation with the immune response for some strains.
Age is known to affect the immune response to influenza vaccination. Adults aged≥65 years produce weaker immune response to vaccination than adults aged <65 years. A number of protective immune functions decline with age, and along with physiological and anatomical changes, this contributes to increased susceptibility to infectious diseases and suboptimal immune responses to vaccination [19]. In the present study, subjects aged≥65 years showed similar immune responses to subjects aged <65 years. The relatively good immune response in subjects aged≥65 years is probably related to a higher rate of influenza vaccination during the previous epidemic season. According to Lim et al.'s study [20], influenza vaccination coverage among subjects aged ≥65 years in South Korea, during the 2004-2005 influenza seasons, was 77.2%. However, in healthy younger adults, the vaccination coverage was only approximately 33% [20].
Data on the correlations between the immunogenicity of influenza vaccines and WBC count or lymphocyte count are often conflicting. Chisholm et al. [21] reported that a response to influenza antigen was not affected by the total WBC count at immunization. Other reports have demonstrated a positive correlation between the response to influenza antigen and total numbers of circulating lymphocytes or neutrophils on the day of immunization [22]. In this study, the WBC and lymphocyte counts did not affect the response to influenza antigen, but ANC affected the response to B influenza antigen.
In this study, total chemotherapy duration showed not a negative, but a positive correlation with the immune response for some strains. Although the reason why it showed a positive correlation between total chemotherapy duration and immune response will be further investigated or verified, the fact that at least the long term chemotherapy in colorectal cancer subjects did not impair an immune response of influenza vaccination, probably due to relatively weaker chemo intensity of colorectal cancer than other cancer, offer a good reason to recommend influenza vaccination to colorectal cancer patients under chemotherapy.
In the present study, influenza vaccine induced an acceptable but limited response in immunocompromised individuals. Therefore, other strategies are needed to reinforce the efficacy of influenza vaccination in immunocompromised patients. Family member vaccination, healthcare worker vaccination, 2-dose vaccination, and avoidance of immunization at times of leukopenia or lymphopenia, might be effective. Because children play an important role in the transmission of influenza virus, all children in a cancer patient's family should be vaccinated [23,24]. However, further studies in this respect are needed.
The lack of an appropriate control group is a limitation of this study; hence, comparison with diabetes mellitus patients was conducted (Table 5). The small sample size is another limitation of this study. Further studies with larger sample sizes that assess the correlation between the immunogenicity of influenza vaccines and chemotherapy state, chemotherapy regimen, and time from cessation of chemotherapy are necessary.
Go to : 
Acknowledgments
This study was supported by the Clinical Radiological Research Project, Korea Institute of Radiological and Medical Sciences (Grant No. 50683-2009).
The specimens used for this study were distributed by the KIRAMS Radiation Biorepository (KRB) according to the recommended procedure.
Go to : 
References
1. Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis. 2006; 194(Suppl 2):S133–S138. PMID: 17163386.

2. Ljungman P, Andersson J, Aschan J, Barkholt L, Ehrnst A, Johansson M, et al. Influenza A in immunocompromised patients. Clin Infect Dis. 1993; 17:244–247. PMID: 8399875.

3. Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C, et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010; 10:521–526. PMID: 20620116.

4. Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009; 9:493–504. PMID: 19628174.

5. Beck CR, McKenzie BC, Hashim AB, Harris RC, Zanuzdana A, Agboado G, et al. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis from a public health policy perspective. PLoS One. 2011; 6:e29249. PMID: 22216224.

6. Cho BH, Kolasa MS, Messonnier ML. Influenza vaccination coverage rate among high-risk children during the 2002-2003 influenza season. Am J Infect Control. 2008; 36:582–587. PMID: 18926312.

7. Meidani M, Rostami M, Dehghani F. Why coverage of influenza vaccine is not enough in patients receiving chemotherapy? Int J Prev Med. 2011; 2:186–187. PMID: 21811662.
8. Muller D, Nguyen-Van-Tam JS, Szucs TD. Influenza vaccination coverage rates in the UK: a comparison of two monitoring methods during the 2002-2003 and 2003-2004 seasons. Public Health. 2006; 120:1074–1080. PMID: 17027881.

9. Earle CC. Influenza vaccination in elderly patients with advanced colorectal cancer. J Clin Oncol. 2003; 21:1161–1166. PMID: 12637485.

10. Loulergue P, Mir O, Alexandre J, Ropert S, Goldwasser F, Launay O. Low influenza vaccination rate among patients receiving chemotherapy for cancer. Ann Oncol. 2008; 19:1658. PMID: 18662953.

11. Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012; 44:25–31. PMID: 22500157.

12. The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Proprietary Medicinal Products (CPMP). Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). London: European Agency for the Evaluation of Medicinal Products;1997.
13. Puthillath A, Trump DL, Andrews C, Bir A, Romano K, Wisniewski M, et al. Serological immune responses to influenza vaccine in patients with colorectal cancer. Cancer Chemother Pharmacol. 2011; 67:111–115. PMID: 20204362.

14. Anderson H, Petrie K, Berrisford C, Charlett A, Thatcher N, Zambon M. Seroconversion after influenza vaccination in patients with lung cancer. Br J Cancer. 1999; 80:219–220. PMID: 10389999.

15. Mazza JJ, Yale SH, Arrowood JR, Reynolds CE, Glurich I, Chyou PH, et al. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005; 3:214–220. PMID: 16303886.

16. Looijmans-Van den Akker I, Verheij TJ, Buskens E, Nichol KL, Rutten GE, Hak E. Clinical effectiveness of first and repeat influenza vaccination in adult and elderly diabetic patients. Diabetes Care. 2006; 29:1771–1776. PMID: 16873778.
17. Xie H, Jing X, Li X, Lin Z, Plant E, Zoueva O, et al. Immunogenicity and cross-reactivity of 2009-2010 inactivated seasonal influenza vaccine in US adults and elderly. PLos One. 2011; 6:e16650. PMID: 21304946.

18. Schafer AI, Churchill WH, Ames P, Weinstein L. The influence of chemotherapy on response of patients with hematologic malignancies to influenza vaccine. Cancer. 1979; 43:25–30. PMID: 761165.

19. Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009; 333:413–429. PMID: 19768417.

20. Lim J, Eom CS, Kim KH, Kim S, Cho B. Coverage of influenza vaccination among elderly in South Korea: a population based cross sectional analysis of the season 2004-2005. J Korean Geriatr Soc. 2009; 13:215–221.

21. Chisholm JC, Devine T, Charlett A, Pinkerton CR, Zambon M. Response to influenza immunisation during treatment for cancer. Arch Dis Child. 2001; 84:496–500. PMID: 11369567.

22. Gross PA, Lee H, Wolff JA, Hall CB, Minnefore AB, Lazicki ME. Influenza immunization in immunosuppressed children. J Pediatr. 1978; 92:30–35. PMID: 619076.

23. Fox JP, Hall CE, Cooney MK, Foy HM. Influenzavirus infections in Seattle families, 1975-1979. I. Study design, methods and the occurrence of infections by time and age. Am J Epidemiol. 1982; 116:212–227. PMID: 7114033.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download