Abstract
Purpose
A population-based study was conducted in order to examine the characteristics of family members of cancer patients in comparison with the general population and also to evaluate the psychosocial impact of cancer patients on their family members.
Materials and Methods
From the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) (2007-2009) dataset, we identified 460 cancer patients and then selected family members of these patients who were aged 20 years or older (n=565). The control group was sampled from members of families without a cancer patient with matching for sex and age (n=2,260). Serial conditional logistic regression models were used for comparison of characteristics between family members of cancer patients and subjects in the control group.
Results
Family members of cancer patients were less employed (57.9% vs. 63.0%, p<0.001), more functionally limited (20.2% vs. 16.5%, p=0.032), and had lower self-rated health (p=0.023) compared with sex and age-matched control subjects. They also had a significantly higher level of stress (79.7% vs. 76.1%, p=0.008), history of depression (12.9% vs. 10.2%, p=0.035), and current depressive symptoms (5.5% vs. 3.5%, p=0.038). However, higher physical activity was reported in family members of cancer patients (13.6% vs. 9.6%, p=0.003) than in control subjects. The presence of a cancer patient in the family showed an association with current depressive symptoms (odds ratio, 1.62; 95% confidence interval, 1.05 to 2.48; p=0.028), however, the association was no longer significant after adjustment for household income, education level, and employment status (p=0.304).
A continuous increase in cancer incidence and decrease in cancer mortality have resulted in a rising number of people living with cancer. As cancer patients live longer, psychological wellbeing of both cancer patients and their family members is becoming a growing concern. Previous studies have focused on the psychological status of cancer patients, and evaluated sociodemographic variables that make cancer patients more susceptible to depression and increased anxiety [1,2]. With the continuous increase in the number of cancer patients and their family members, knowing which factors contribute to the burden of family members is critical. To date, many studies have been conducted for evaluation of the burden of caregivers in chronic diseases such as stroke and dementia, however, the burden of family members of cancer patients has not been evaluated in depth [3-5]. Meta-analyses have evaluated the burden of caregivers alone, however, socioeconomic status (SES), physical function, perceived health status, psychological factors, health behavior, and health-related quality of life (HRQOL) of the family members in a household have not been investigated [6,7].
In a recent study reported by Palos et al. [8], who assessed the risk of caring for underserved patients with advanced cancer, sadness and distress were more prevalent among caregivers compared with cancer patients, and approximately 40% of caregivers were found to be at an increased risk for moderate to severe sadness and distress. This finding suggests that more attention is required for family members, and knowing how to support them is imperative.
Economic burden and financial distress are important issues that affect treatment strategy in long-term cancer survivorship. With newly emerging diagnostic and treatment patterns, expenditures on cancer treatment have increased and are expected to show a continuous increase in the future [9]. All of the factors mentioned above may contribute to greater anxiety, depression, poorer social well-being, and lower HRQOL.
In this study, we compared psychological, socioeconomic, physical function, health behavioral, and HRQOL-related aspects of family members of cancer patients and control subjects. We then performed an evaluation with an emphasis on factors that contribute to psychosocial wellbeing, such as level of stress, history of depression, and current depressive symptoms in all family members of each cancer patient.
The Ministry of Health and Welfare of Korea began conducting the Korea National Health and Nutrition Examination Surveys (KNHANES) in 1998 in order to examine the general health and nutrition status of Koreans. KNHANES IV was conducted from July 2007 to December 2009. The survey used a stratified multistage probability sampling design for the South Korean population [10]. The uniqueness of this study is that the survey was based on households, and every member of the household was required to complete the survey. KNHANES consisted of four different measures: a health interview, health behavior survey, health examination, and a nutrition survey. In KNHANES IV, 31,705 individuals aged>1 year were invited to participate in the health interview and examination (6,455 in 2007, 12,528 in 2008, and 12,722 in 2009), and 24,871 individuals participated in the survey (4,594 in 2007, 9,744 in 2008, and 10,533 in 2009) at a response rate of 78.4% (71.2% in 2007, 77.8% in 2008, and 82.8% in 2009). Participants who reported a history of cancer (n=460) were considered cancer patients and were excluded from the analysis. Participants under age 19 (n=6,465) were excluded because they were not required to complete questionnaires for HRQOL. We defined 'family members of cancer patients' as individuals who have at least one cancer patient within his/her family. Participants who do not live with cancer patients were considered as candidates for the control group. Because there was a significant age difference between family members of cancer patients (n=565) and non-cancer family members (n=17,351), we sampled the control group with 1:4 individual matching on sex and age. Therefore, the final study population included 565 family members of cancer patients and 2,260 matched control subjects (Fig. 1).
Information on demographic and SES, including sex, age, household income, education, and employment was obtained using self-administered questionnaires. Families were divided into four groups according to monthly household income (lowest, lower intermediate, higher intermediate, and highest groups) and education level as four groups (primary [≤6 years of schooling], middle [6-9 years of schooling], high [9-12 years of schooling], and college [≥13 years of schooling]). Employment status was reported as either yes or no. Physical function was categorized according to two groups: limited in anyway and not limited. Participants were asked if they had ever had comorbidities such as hypertension, heart disease, stroke, arthritis, or chronic renal disease. They were also asked to report self-rated health as very good, good, fair, poor, or very poor. For our analyses, self-rated health was regrouped into three categories as very good to good, fair, or poor to very poor.
For evaluation of psychological factors, respondents were asked to report their level of stress, history of depression, and current depressive symptoms. The level of stress was reported as none/small or some/extreme. Respondents reported history of depression and current depressive symptoms as yes or no.
Health related behavioral risk factors were measured using self-reported questionnaires. Smoking status was categorized according to two groups: never smoker/ex-smoker and current smoker. Current alcohol use (average consumption) was measured by obtaining information related to respondents' self-reported alcohol behavior. Specifically, frequency of alcohol consumption was used as the measure of alcohol status: less than once per month, once per month, 2-4 times per month, 2-3 times per week, and more than four times per week. For our analyses, alcohol consumption was coded as yes if respondents reported consuming alcohol≥2 times/wk. Otherwise, alcohol consumption was coded as none [11]. Physical activity was measured using the frequency of moderate and vigorous physical activity per week. For analyses, recommended physical activity level was coded as yes if participants reported more than two days of moderate activity per week. Otherwise, physical activity level was coded as none for those who reported physical activity less than two days per week.
HIQOL was measured using the EuroQol, which consists of the health-status descriptive system (EQ-5D) and a visual analogue scale (EQ-VAS). The EQ-5D records the level of self-reported problems according to five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) [12,13]. Each of the dimensions is assessed based on a single question with three response levels (no problem, some problems, and extreme problems). Using the combination of these items, a single health index was calculated using the Korea valuation set developed by the Korean Centers for Disease Control and Prevention [14]. Scores on the EQ-5D index range from -0.171 to 1, where 1 indicates no problem in any of the five dimensions, zero indicates death, and negative values indicate a health status worse than death. Next, respondents described their own health status using a VAS ranging from 0 (worst imaginable health) to 100 (best imaginable health), which is represented as EQ-VAS. Eighty five percent of participants responded to EQ-5D and EQ-VAS.
Descriptive statistics and χ2 tests were used for examination of demographic characteristics and differences between the family members of cancer patients and subjects in the control group. The survey year was also included as a controlled variable because the EQ-5D and EQ-VAS scores differed significantly according to survey year (data not shown). Conditional logistic regression analysis was performed, and presence of a cancer survivor, household income, education level, and employment status were dependent variables. All statistical analyses were performed using SAS ver. 9.2 (SAS Institute, Cary, NC) and p<0.05 was considered statistically significant.
Characteristics of family members of cancer patients and subjects in the control group are shown in Table 1. We excluded cancer patients from the analysis because our aim was to perform a comparison of the psychosocial status of family members only. The mean age was 51.2±17.6 years. No significant differences in household income and education level were observed between the two groups, although the group of family members of cancer patients had more respondents with lower household income and education level. Employment rates differed significantly among the two groups, with fewer family members of cancer patients currently employed (57.9% vs. 63.0%). Family members of cancer patients reported a significantly higher rate of functional limitation (20.2% vs. 16.5%). Prevalence of comorbidity was similar between the two groups (p for difference=0.091), however, family members of cancer patients were more likely to report their health status as poor to very poor (24.4% vs. 20%). Significantly higher levels of stress (79.7% vs. 76.1%), history of depression (12.9% vs. 10.2%), and current depression (5.5% vs. 3.5%) were reported by family members of cancer patients. Results for health behavioral risk factors, cigarette smoking, and alcohol consumption were similar; however, physical activity was more common in family members of cancer patients than in the control group (13.6% vs. 9.6%). No differences in mean EQ-5D (p for difference=0.361) and EQ-VAS scores (p for difference=0.900) were observed between the two groups.
Significant difference in psychological and cognition status was observed between family members of cancer patients and the general population. Family members of cancer patients reported a relatively higher level of stress and more past or current depression. We performed univariate and multivariate analyses for determination of which factors would have a greater impact on psychosocial status of family members. In univariate analyses, presence of a cancer patient significantly increased the risk of current depression (odd ratio [OR], 1.62; 95% confidence interval [CI], 1.05 to 2.48). Lower education level (primary school) showed a significant association with history of depression (OR, 1.99; 95% CI, 1.21 to 3.27) and current depression (OR, 3.51; 95% CI, 1.52 to 8.11). Household income and employment status did not show an association with stress, history of depression, and current depressive symptoms (Table 2). However, after adjustment for household income, education level, and employment status, presence of a cancer patient did not show a significant association with stress and depression. In the multivariate adjusted models, only low education showed a significant association with past (OR, 2.11; 95% CI, 1.09 to 4.05) and current (OR, 3.61; 95% CI, 1.19 to 10.89) depressive symptoms (Table 3).
The current study evaluated the psychosocial impact of cancer patients on their family members in a national representative survey. Family members of cancer patients experienced relatively higher levels of stress and depressive symptoms. They were also less employed and more functionally limited. The presence of cancer patients in a family showed an association with current depressive symptoms; however, after adjustment for household income, education level, and employment status, the association was no longer significant. To the best of our knowledge, this is the first study to assess the psychological, socioeconomic, and physical burden of caregivers using family-based questionnaires.
Cancer is a stressful event for both patients and family members [14]. After the diagnosis of cancer, treatment of cancer and follow-up management are shared among family members. Family members are often expected to deal with cancer-related symptoms and perform clinical tasks. As a result of patients' pre-existing comorbidities, family members of elderly cancer patients face a highly complex set of challenges. In addition, because a large portion of cancer treatment is administered in ambulatory settings, family members are becoming more responsible for providing care [15].
Previous studies have reported that having a cancer patient in a family can be a distress. In a study reporting on psychosocial status and quality of life of patients and spouses in patients with prostate cancer, spouses had the lowest emotional quality of life of all participants, suggesting that cancer takes an emotional toll on spouses [16]. Another study examined levels of depression and anxiety in newly diagnosed adult patients and their adult family members. Family members had higher levels of depression and anxiety than cancer patients [17]. Findings of our study also showed that a diagnosis of cancer impacts on family caregivers from a psychological perspective. Significantly higher levels of stress, history of depression, and current depressive symptoms were observed in family members of cancer patients than in control subjects.
We examined multiple factors representing SES because low SES may be an indication that individuals to live in poorer conditions, have less access to healthcare, and experience greater psychological stress. Our results showed that those with lower household income were more likely to experience depression, although the significance was not met. Low SES is a substantial adverse prognostic factor and a risk factor for all-cause mortality after diagnosis of cancer; therefore, disparities in SES may influence both mental and physical wellbeing and should be considered in evaluation of psychosocial distress associated with cancer [18-20].
With respect to health behavioral risk factors, high occurrence of current smoking among family members of cancer patients draws attention. According to Burke et al. [21], less than half of smoking cancer patients quit smoking after their cancer diagnosis, and only 62% of smoking cancer patients received smoking cessation counseling from their physicians. Although no study on smoking behavior of family members has been reported, effective promotion of smoking cessation programs is required for secondary cancer prevention. Oncologists and primary care physicians should provide guidance on health promotion interventions to both cancer patients and family members [22].
The strength of our study is that these data were obtained from a nationwide population and therefore provided representative information on the Korean population. In addition, this is the first large-scaled questionnaire study based on households. Nevertheless, several study limitations should be considered. First, due to the cross-sectional nature of the data, determination of the causal relationship between cancer diagnosis and the identified parameters is difficult. Cancer treatment itself can cause family members to forgo their jobs, leading to lower household income, or it may be that diagnosis of cancer is more likely in populations with low SES [23]. Therefore, conduct of long-term follow-up studies will be needed for evaluation of the causal relationship between socioeconomic and psychological burdens of family members. Second, the findings of this study were based on self-reported health status, thus, there is a possibility of inaccurate reporting or not answering certain questions. Depression was also assessed using the self-reported questionnaire rather than being diagnosed by a doctor; therefore, it may not provide an accurate indication of the prevalence of clinically relevant depression. In addition, depression may result from other chronic comorbidities, such as stroke and dementia, which should be considered in future studies. Third, we did not differentiate cancer patients who were under active treatment from those who were not. Cancer patients who are under active treatment and those who are not may have different impact on the psychosocial status of their family members; however, we were not able to perform further investigation on this matter. Finally, due to the retrospective nature of this study, we were not able to determine the relationship between caregiver and patient and whether the caregiver was living in the same household or not.
Despite these limitations, the results of the current study suggest that more concern for family members is needed, as cancer may influence the whole family, not just the patient. According to findings of recent studies, caregivers who received intervention reported significantly less burden, less depression, and less distress, compared to those who did not. They also reported more knowledge, better coping, and greater mental well-being [24,25]. Therefore, there is a need for conduct of more effective studies with cancer caregivers or patient-caregiver dyads for implementation of evidence-based interventions in clinical settings. Healthcare professionals should plan risk assessment of caregiver's capacity and assist them in solving practical problems that arise as a result of the cancer diagnosis.
As the number of people living with a history of cancer continues to increase, identification of optimal methods for promoting the psychological health and well-being of both cancer patients and their family members is essential. Family members, who are somewhat invisible to the healthcare team, should be recognized for their mental and physical well-being. Assessment of stress and psychological distress should be followed by guidance and individualized interventions for attenuation of the health consequences.
References
1. Luckett T, Goldstein D, Butow PN, Gebski V, Aldridge LJ, McGrane J, et al. Psychological morbidity and quality of life of ethnic minority patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2011; 12:1240–1248. PMID: 21996168.

2. Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, Cleeland CS. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society's Studies of Cancer Survivors. Cancer. 2011; 117:2779–2790. PMID: 21495026.

3. Collins LG, Swartz K. Caregiver care. Am Fam Physician. 2011; 83:1309–1317. PMID: 21661713.
4. Bevans M, Sternberg EM. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA. 2012; 307:398–403. PMID: 22274687.

5. Kim Y, Schulz R. Family caregivers' strains: comparative analysis of cancer caregiving with dementia, diabetes, and frail elderly caregiving. J Aging Health. 2008; 20:483–503. PMID: 18420838.
6. Hagedoorn M, Buunk BP, Kuijer RG, Wobbes T, Sanderman R. Couples dealing with cancer: role and gender differences regarding psychological distress and quality of life. Psychooncology. 2000; 9:232–242. PMID: 10871719.

7. Hodges LJ, Humphris GM, Macfarlane G. A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Soc Sci Med. 2005; 60:1–12. PMID: 15482862.

8. Palos GR, Mendoza TR, Liao KP, Anderson KO, Garcia-Gonzalez A, Hahn K, et al. Caregiver symptom burden: the risk of caring for an underserved patient with advanced cancer. Cancer. 2011; 117:1070–1079. PMID: 20960510.

9. Weaver KE, Rowland JH, Bellizzi KM, Aziz NM. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the United States. Cancer. 2010; 116:3493–3504. PMID: 20549763.
10. Choi YJ, Kim HC, Kim HM, Park SW, Kim J, Kim DJ. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998-2005. Diabetes Care. 2009; 32:2016–2020. PMID: 19675201.
11. World Health Organization. International guide for monitoring alcohol consumption and related harm. Geneva: World Health Organization;2000.
12. The EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990; 16:199–208. PMID: 10109801.
14. Chen X, Fan R. The family and harmonious medical decision making: cherishing an appropriate Confucian moral balance. J Med Philos. 2010; 35:573–586. PMID: 20855426.

15. Given BA, Given CW, Sherwood PR. Family and caregiver needs over the course of the cancer trajectory. J Support Oncol. 2012; 10:57–64. PMID: 22222251.

16. Northouse LL, Mood DW, Montie JE, Sandler HM, Forman JD, Hussain M, et al. Living with prostate cancer: patients' and spouses' psychosocial status and quality of life. J Clin Oncol. 2007; 25:4171–4177. PMID: 17635953.

17. Edwards B, Clarke V. The psychological impact of a cancer diagnosis on families: the influence of family functioning and patients' illness characteristics on depression and anxiety. Psychooncology. 2004; 13:562–576. PMID: 15295777.

18. Meropol NJ, Schrag D, Smith TJ, Mulvey TM, Langdon RM Jr, Blum D, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009; 27:3868–3874. PMID: 19581533.

19. Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008; 113:582–591. PMID: 18613122.
20. Boyd C, Zhang-Salomons JY, Groome PA, Mackillop WJ. Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol. 1999; 17:2244–2255. PMID: 10561282.

21. Burke L, Miller LA, Saad A, Abraham J. Smoking behaviors among cancer survivors: an observational clinical study. J Oncol Pract. 2009; 5:6–9. PMID: 20856708.

22. McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control. 2003; 10:325–333. PMID: 12915811.

23. Mackillop WJ, Zhang-Salomons J, Boyd CJ, Groome PA. Associations between community income and cancer incidence in Canada and the United States. Cancer. 2000; 89:901–912. PMID: 10951356.

24. Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin. 2010; 60:317–339. PMID: 20709946.

25. Northouse L, Williams AL, Given B, McCorkle R. Psychosocial care for family caregivers of patients with cancer. J Clin Oncol. 2012; 30:1227–1234. PMID: 22412124.

Fig. 1
A flow chart showing recruitment of the study population. In Korea National Health and Nutrition Examination Surveys IV, 31,705 individuals aged>1 year were sampled by the health interview. From the initial sample of 31,705 individuals, 6,834 individuals did not complete the survey and participants under 19 (n=6,465) were excluded because they did not complete questionnaires for health-related quality of life; 460 cancer patients were excluded from the study. A total of 565 family members of cancer patients and 17,351 members of the general population were age-, sex-matched to 1 to 4. The final study population included 565 family members of cancer patients and 2,260 matched control subjects.
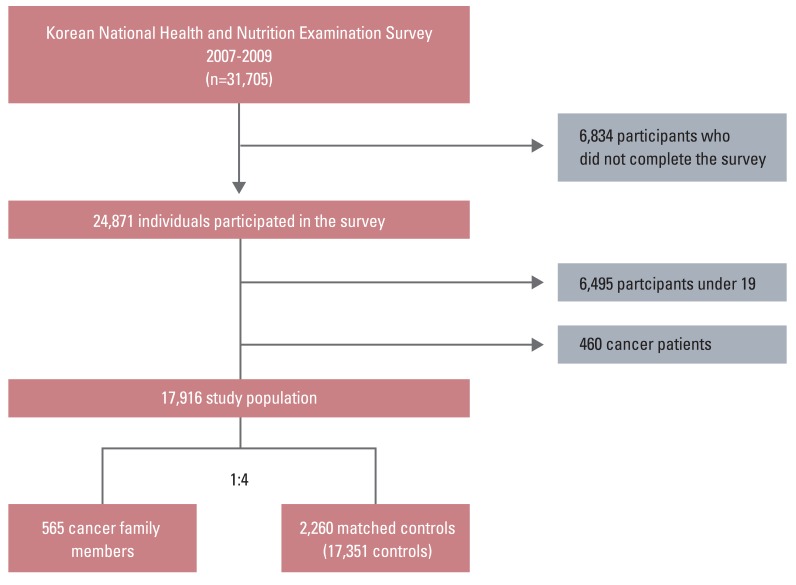
Table 1
Psycho-socio-physical status of family members of cancer patients and control subjects (1:4 matched)
| Cancer family members (n=565) | Controls (n=2,260) | p-value | |
|---|---|---|---|
| Gender | |||
| Male | 298 (52.7) | 1,192 (52.7) | NA |
| Female | 267 (47.3) | 1,068 (47.3) | |
| Age | 51.2±17.6 | 51.2±17.6 | NA |
| Socioeconomic factors | |||
| Quartile of household income | 0.098 | ||
| Lowest | 136 (24.1) | 457 (20.2) | |
| Lower intermediate | 148 (26.2) | 540 (23.9) | |
| Higher intermediate | 111 (19.6) | 522 (23.1) | |
| Highest | 158 (28.0) | 643 (28.5) | |
| Unknown | 12 (2.1) | 98 (4.3) | |
| Education level | 0.340 | ||
| Primary | 160 (28.3) | 587 (26.0) | |
| Middle | 63 (11.2) | 230 (10.2) | |
| High | 155 (27.4) | 702 (31.1) | |
| College | 147 (26.0) | 602 (26.6) | |
| Unknown | 40 (7.1) | 139 (6.1) | |
| Employed (yes) | 327 (57.9) | 1,423 (63.0) | <0.001 |
| Physical function and health status | |||
| Functional limitation | 0.032 | ||
| Limited in any way | 114 (20.2) | 373 (16.5) | |
| Not limited | 411 (72.7) | 1,750 (77.4) | |
| Unknown | 40 (7.1) | 137 (6.1) | |
| Comorbiditiesa) | 0.091 | ||
| No | 330 (58.4) | 1,297 (57.4) | |
| Yes | 32 (5.7) | 92 (4.1) | |
| Unknown | 203 (35.9) | 871 (38.5) | |
| Self-rated health | 0.023 | ||
| Very good to good | 226 (40.0) | 871 (38.5) | |
| Fair | 161 (28.5) | 800 (35.4) | |
| Poor to very poor | 138 (24.4) | 453 (20.0) | |
| Unknown | 40 (7.1) | 136 (6.1) | |
| Psychological factors | |||
| Level of stress | 0.008 | ||
| Some or extreme | 420 (79.7) | 1,719 (76.1) | |
| None or small | 107 (19.0) | 409 (18.1) | |
| Unknown | 38 (1.3) | 132 (5.8) | |
| History of depression | 73 (12.9) | 231 (10.2) | 0.035 |
| Current depression | 31 (5.5) | 80 (3.5) | 0.038 |
| Health behavioral risk factors | |||
| Current smoker | 92 (16.3) | 348 (15.4) | 0.322 |
| Alcohol consumption (≥2/wk, %) | 126 (22.3) | 500 (22.1) | 0.441 |
| Physical activity | 77 (13.6) | 218 (9.6) | 0.003 |
| Quality of life | |||
| EQ-5D | 0.92±0.13 | 0.93±0.14 | 0.361 |
| EQ-VAS | 73.7±17.3 | 72.9±17.9 | 0.900 |
Table 2
Univariate (unadjusted) analyses of factors influencing psychosocial status of the study population
Table 3
Multivariate (adjusted) analyses of factors influencing psychosocial status of the study population




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download