A 27-year-old man was admitted with a palpable abdominal mass in May 2009. Chest and abdominopelvic computed tomography (CT) scan showed enlarged para-aortic lymph nodes measuring 9 cm in size, including supraclavicular, mediastinal, and abdominal lymph nodes. Multiple liver and lung metastases were also observed. Levels of serum beta-human chorionic gonadotrophin (beta-hCG), alpha-fetoprotein (AFP), and serum lactate dehydrogenase (LDH) were elevated (beta-hCG, 175,910 mIU/mL; AFP, 109.5 ng/mL; LDH, 475 IU/L). A positron emission tomography (PET)-CT scan showed multiple areas of strong fluorodeoxyglucose (FDG) uptake in the aforementioned lesions. Percutaneous lung biopsy for metastasis confirmed the histological diagnosis of seminoma. Ultrasonography of the testis showed a diffuse infiltrative lesion of the right testis. The patient underwent right orchiectomy. Pathologic examination also revealed a seminoma. Although the diagnosis of seminoma was confirmed in lung and orchiectomy specimens, because of the elevated level of AFP, it was considered to be mixed germ cell tumor (GCT) with seminoma. Based on chest CT, abdominopelvic CT, PET scan, and tumor markers, the patient's state corresponded to stage IIIC and the high risk group according to the American Joint Committee of Cancer (AJCC) staging system and International Germ Cell Cancer Collaborative Group (IGCCCG) classification. After undergoing right orchiectomy, he received primary chemotherapy with bleomycin, etoposide, and cisplatin (BEP). After four cycles of BEP (July 2009), on the subsequent chest and abdominopelvic CT scan, metastatic nodules in the lung and liver had almost disappeared. However, the mediastinal and para-aortic lymph nodes, which had obviously decreased in size, were still evident. The largest residual mass was a para-aortic lymph node, which was seen as a thin-walled, cystic mass, measuring 3.2 cm×2.3 cm. A follow-up PET-CT scan showed no FDG uptake in the residual masses. Levels of beta-hCG, AFP, and LDH were normalized. According to the guidelines, surgical resection should be performed at all sites of residual mass. However, due to the presence of multiple lesions, which were scattered throughout the thorax and abdomen, removal of all residual masses was impossible. Therefore, the patient received two additional cycles of etoposide and cisplatin (EP) and the treatment was completed. Chest CT, abdomen CT, and PET-CT showed no significant change in the residual masses and no newly developed lesions were observed during the following year. At 16 months after chemotherapy (December 2010), a mass in the left supraclavicular area was observed on follow-up neck CT. The mass was a well-defined enhancing lesion with an internal necrotic or cystic component measuring 3×2.2×2 cm in size (
Fig. 1A). This lesion did not show FDG uptake on PET-CT (
Fig. 1B). The other residual masses had decreased in size. The levels of all tumor markers were consistently within normal limits (beta-hCG, <0.1 mIU/mL; AFP, 3.3 ng/mL; LDH, 171 IU/L). The supraclavicular mass, which had been growing for the last three months, was suspected of being metastatic and was surgically resected. Histological examination revealed a subcutaneous, fibrohistiocytic proliferation with cystic spaces and a pseudocapsule, surrounded by lymphoid tissue (
Fig. 2A). At high power, the tumor cells were spindle or epithelioid in shape, and were arranged in a nodular pattern (
Fig. 2B). In immunohistochemical staining, the tumor cells were positive for CD68 (
Fig. 2C) and desmin (focal) (
Fig. 2D), and negative for epithelial membrane antigen, S-100, CD34, and human melanoma black 45. Based on the morphology and results of immunohistochemical staining, a diagnosis of AFH was made. He did not receive subsequent chemotherapy or radiation therapy. Relapse of the tumor did not occur within 12 months.
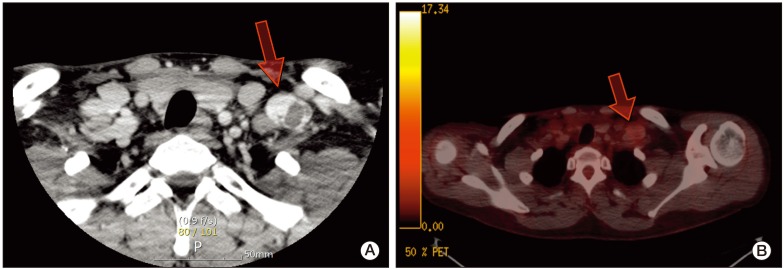 | Fig. 1(A) Enhanced neck computed tomography showed a well-defined enhancing mass with an internal cystic component in the left supraclavicular area (arrow). (B) Positron emission tomography-computed tomography showed no definite fluorodeoxyglucose uptake in the lesion (arrow). 
|
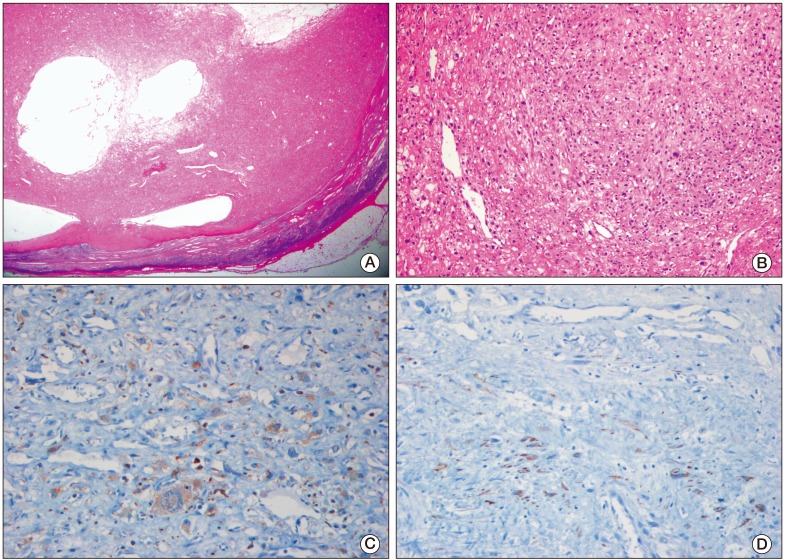 | Fig. 2(A) Low magnification microscopic photograph of the tumor demonstrated fibrohistiocytic proliferation with cystic spaces and a pseudocapsule, surrounded by lymphoid tissue (H&E staining, ×10). (B) The tumor cells were spindle or epithelioid in shape, and were arranged in a nodular pattern (H&E staining, ×100). (C) The tumor cells were positive for CD68 (×200). (D) The tumor cells were focally positive for desmin (×200). 
|






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download