Abstract
Stereotactic body radiation therapy (SBRT) is a newly developed technique currently in clinical use. SBRT originated from stereotactic radiosurgery for intracranial tumors. SBRT has been widely used clinically for lung cancer. The practice of SBRT demands different kinds of patient fixation, breathing control, target determination, treatment planning, and verifications. The history and current standard technique are reviewed. Clinical studies of lung cancer showed high local control rates with acceptable toxicities. Past and on-going clinical trials are reviewed.
Stereotactic radiotherapy (SRT) is method to deliver highly precise radiation to intracranial tumors that can maintain fixed precision within 1-2 mm. This treatment method aims to minimize the radiation dose administered to normal tissues and increase the radiation administered to the tumor by accurately focusing radiation onto the target lesion (hereafter "target"). SRT was originally developed for clinical application in the 1960s as "gamma knife radiosurgery," most commonly employing single high dose radiation, and evolved to the current form after linear accelerator (LINAC)-based radiosurgery was introduced by 1983. Both techniques initially were developed for treating intracranial brain tumors. The application of the stereotactic technique on the trunk of the body was initiated in the 1990s. In the USA, this is known as stereotactic body radiation therapy (SBRT) or stereotactic ablative external radiotherapy, while in Europe, it is known as extracranial stereotactic radiotherapy. In Japan it is commonly known as pin-pointed radiotherapy. Its first recognized use was by Blomgren et al. [1] of the Karolinska Institute in 1991, while in Japan, it was implemented by Uematsu et al. [2] in 1994. However, the main problem with performing SBRT on tumors of the trunk as opposed to brain tumors was tumor movement caused by bodily and respiratory movements. Therefore, when performing SBRT for tumors of the body trunk, it is extremely important to establish accurate patient fixation, breathing control systems, and verification of the irradiation field prior to each treatment.
To restrain the bodies of patients during treatment, it is important to have suitable fixation methods. Various vacuum contact-type braces are currently available for use in SBRT that involve styrofoam in a plastic body frame.
In lung tumors, tumor movement during respiration cannot be ignored. Methods that deal with the respiratory movement of patients can be broadly divided into the breath holding technique, the restricted respiration technique (abdominal compression), and respiratory gating technique. Efforts to reduce tumor movement during respiration (internal margin) are required.
The breath holding technique is a voice or light-gated intermittent irradiation system in which the patient temporarily suspends his/her breathing following an audible or light signal, allowing for irradiation. In this technique, breath is generally held at the resting expiratory level, and theoretically internal tumor volume, a radiation volume including tumor motion, is minimized, making it possible to set the minimal irradiated volume. In addition, devices can be used to help suspend breathing [3].
An alternative to this is the restricted respiration technique in which major movements of the patient's diaphragm are restricted by compressing the upper abdomen using either a belt or plate-like brace. Tumor movement is ascertained by fluoroscopy, and if found to be greater than 8-10 mm, it is common to restrict movement [4]. In practice, restriction of respiration is required owing to tumor movement greater than 8-10 mm in less than 25% of all patients.
In contrast to these methods, a respiratory gating technique has been developed that synchronizes irradiation with the respiratory phase (mainly the expiratory phase) while the patient breathes freely but periodically. This is a frequently used technique in which sensors are attached to the thoracic wall of the patient, or gold markers are introduced into the tumor [5], and irradiation is performed while sensing the patient's breathing.
In highly accurate treatment planning, using CT images usually taken at 1-3-mm intervals, radiation oncologists delineate the contour of the tumor and the organs at risk. The breath synchronization and breath holding methods mentioned above are a prerequisite of treatment, and CT images are obtained in accordance with these techniques. When the restricted respiration technique is employed, a prolonged or slow scan CT imaging technique is used in which images are obtained slowly, with a scan time of >4 seconds taken per slice to improve the accuracy of irradiation. It is important to be aware that the target definition differs according to these CT imaging techniques.
In recent years, 4-dimensional (4D) CT imaging technology has emerged, which involves placing infrared markers on the body surface of the patient to obtain a respiratory signal. Using this technique, target information (such as maximum intensity projection image) for the entire respiratory phase can be obtained. It is extremely useful in SBRT.
Once the target is delineated using reconstructed 3-dimensional (3D) images such as Beam's eye view or Room's eye view, the radiation field is determined by combining various factors, including the direction and energy of radiation. Non-coplanar 3D conformal multiport irradiation and static multiple arc radiotherapy are often used. In these methods, using either fixed multiport irradiation with >6 ports or rotatory irradiation of >400°, an approximate equivalent dose distribution can be achieved. Target dose in treatment planning include homogeneous radiation dose within the target (within 10%) and reduction (<15%) of lung capacity (V20) of >20 Gy of radiation. It is possible to achieve this by administering 3D irradiation from a total of 6-8 fields, including irradiation at an angle of +/- 20-40° non-coplanar beams as well as coplanar beams to the body's axis (Fig. 1). Calculating the correct 3D radiation dose allowing for inhomogeneity correction is essential, as is the correction of the radiation dose due to the frame. Furthermore, attention should be paid to the radiation dose prescription used. In Japan, the isocenter is most often the radiation dose prescription point, while in Europe and the USA, the marginal dose of radiation at 80-90% is often used. It is important to note that treatment planning may differ according to the radiation field margin and the dose calculation method.
Prior to each dose of irradiation, verification images are created and checked using a high-energy X-ray image, portal image, or in-room CT to confirm that the correct site is being irradiated. In SBRT in particular, it is essential to conduct verification prior to irradiation. To verify the reproducibility of irradiation before each treatment, verification images (confirmation images taken before irradiation using the imaging equipment) are obtained. Reproducibility of the body position with the simulation film during treatment planning is confirmed. As a result, administering irradiation within the usual error range of 2-3 mm is possible through verification of each result before each treatment. In the Japan Clinical Oncology Group (JCOG) 0403 multicenter collaborative clinical trial, a margin of 5 mm is essential. There are an increasing number of facilities that conduct pretreatment verification using either X-ray equipment attached to image-guided radiation therapy (IGRT) or by CT, with equipment set up in the same room as the radiotherapy equipment.
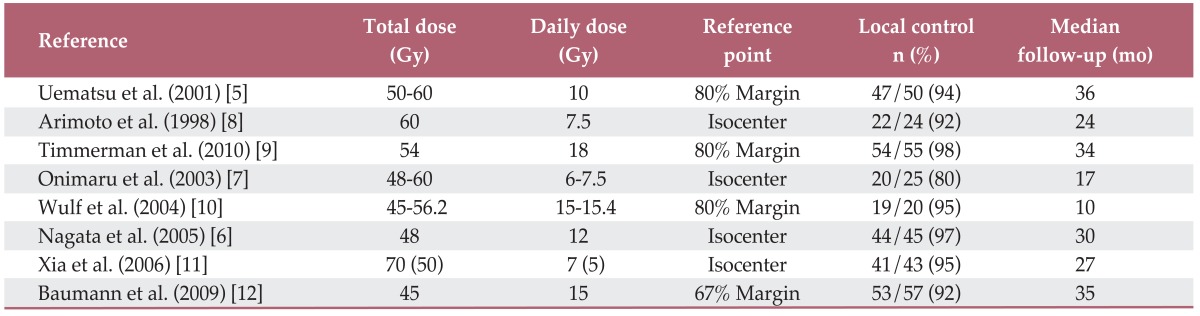
Many different fractionated irradiation techniques have been used: 12 Gy×4 fractions per radiation treatment [6], 10-12 Gy×5-6 fractions per radiation treatment [5], 7.5 Gy×8 fractions per radiation treatment [7], and 15 Gy×3 fractions per radiation treatment. Regardless of the technique used, when the biological effective dose (BED) is >100 Gy, the local control rate is 88-96% with some variation (Table 1) [5-12]. In these different fractionated irradiation methods, the radiation dose, total dose, and number of fractionations are often extrapolated using a linear quadratic (LQ) model calculation based on α/β values. For example, when the α/β value of the tumor is 10, a radiation dose of 12 Gy×4 fractions corresponds to 88 Gy×2 fractions. Using SBRT technology, it is possible to substantially increase the radiation dose.
Fowler [13] demonstrated that the LQ model can be clinically applied with few fractionations and that good localized control can be achieved with a BED>100 Gy. In addition, it is unclear whether the number of radiations in SBRT can ultimately be reduced to one. However, for radiobiological reasons, fractionated irradiation is advantageous as long as hypoxic fractions exist in the tumor. Results from the USA and Europe estimate that 3-5 fractionated irradiations are the minimum number of irradiations while single irradiation is unsatisfactory.
Onishi et al. [14] examined cases from 13 medical facilities throughout Japan and reported treatment outcomes. While the local control rate was 86%, in cases able to undergo irradiation of BED>100 Gy with surgery, the 5-year survival rate was excellent with 90% for the IA stage and 84% for the IB stage [14].
In Europe and the USA, Wulf et al. [10], Timmerman et al. [9], Xia et al. [11], and Baumann et al. [12] all report favorable local effects. However, the number of cases in these papers, when compared to cases in Japan, may have had poorer prognosis because more unfavorable cases were treated as subjects.
In clinical results, the risk of radiation pneumonitis with symptoms greater than Common Terminology Criteria for Adverse Events (CTCAE)-grade 3 is extremely low compared with conventional radiotherapy for stage III lung cancer. In other words, provided an isolated tumor of <4-5 cm in diameter is targeted in a lung, the irradiated volume of normal lung is within the permissible range. The majority of stage I lung cancer cases present asymptomatically, and care must therefore be taken with regard to treatment of emergent complications. However, in the subgroup of patients with poor respiratory function and in particular with underlying interstitial lung disease, there is a risk of fatal radiation pneumonitis (0.5-1.2%), and extra care must be taken [15]. Clinicians should be aware of the risk of other complications, such as rib fracture, intercostals neuralgia, pleural effusion, liver dysfunction, and brachial plexopathy. Furthermore, attention should be paid to extrapulmonary complications with central tumors close to the mediastinum. There have been reports of fatal hemoptysis [16,17] and fatal esophageal ulcers [18]. Appropriate risk management is essential in central lung cancers where irradiation of the mediastinum (heart/large artery, trachea/bronchi, and esophagus) is unavoidable.
In Japan, a multicenter collaborative clinical trial, the "JCOG 0403 phase II clinical trial of SBRT for T1N0M0 non-small cell lung cancer" took place in 15 facilities throughout Japan. The trial evaluated the efficacy and safety of SBRT for T1N0M non-small lung cancer cases in both operable and inoperable cases. On the one hand, with cases unfit for standard surgery, the question is whether current standard therapy with a total dose of 60-70 Gy, at 2 Gy per day should be replaced. On the other hand, where surgery is possible but refused, the question is whether clinical outcomes equal to surgery can be achieved. The primary endpoint is the 3-year survival rate with secondary endpoints being the overall survival rate, the progression-free survival period, the type of recurrence, and adverse events. The treatment method comprises a total radiation dose of 48 Gy at 12 Gy per day per fraction, three or four times per week for a total of four fractions, with the total treatment period within four to eight days. The results of the operable (but rejected) cases, reported in 2010, showed that although majority of subjects were elderly individuals with a mean age of 79 years, the 3-year survival rate was 76% and the 3-year local control rate calculated as per the Radiation Therapy Oncology Group (RTOG) was 86%. There were very few adverse events over grade 3 (6%). In 2012, the results of the inoperable cases, also using elderly subjects with a mean age of 78 years, were reported. Here the 3-year survival rate was 60%; the 3-year local control rate was 88% with no grade 5 adverse events.
The RTOG 0239 results were reported in the USA in 2009. This clinical trial tested 60 Gy by 3 fractions for the purpose of local control in T1-3N0M0 inoperable lung cancers<5 cm in size. The median observation period was 36 months, and there was a high local control rate (recurrence within the planning target volume) of 98% at 3 years. However, although there were no grade 5 adverse events, 4% were grade 4 and 24% were grade 3.
In the USA, studies currently underway include the RTOG 0618 on stereotactic irradiation (excluding partial response cases for local effectiveness) for operable lung cancer, the RTOG 0813 dose escalation study from 10 Gy×5 fractions for central hilar lung cancer, and the RTOG 0915 study comparing 12 Gy×4 fractions against 34 Gy×1. Furthermore, in Europe, a scandinavian stereotactic precision and conventional radiotherapy evaluation (SPACE) trial comparing 15 Gy×3 fractions against 2 Gy×35 fractions is currently underway.
Randomized controlled studies between surgery and SRT, including the commencement of American College of Surgeons Oncology Group (ACOSG)/RTOG trial and the randomized study to compare cyberknife to surgical Resection in stage I non-small cell lung cancer (STARS) trial are underway.
Recent breakthroughs in CT imaging technology paved the way for the discovery of ground glass opacity (GGO) early-stage lesions in the lung. The only current option for such lesions is to obtain a definite diagnosis by surgery. However, it is common for patients with these lesions to be unsuitable for radical surgery because of concurrent lung disease. Studies are needed in which surgery and SBRT are compared for GGO lesions that expand during observation [19].
SBRT is often used for patients with poor respiratory function who are considered unsuitable for surgery. Of these patients, only those with chronic obstructive pulmonary disease are not at high risk of radiation pneumonitis following irradiation; therefore, SBRT is considered suitable. However, there is no consensus on the possibility of SRT for patients with any degree of impaired pulmonary function. An analysis of these adaptive criteria is anticipated. In cases with active interstitial pneumonia, there have been many reports of fatal irradiation pneumonitis, and it is generally accepted that they should be excluded from treatment.
When lung cancer develops near the pulmonary hilum, the treatment raises the risk of radiation exposure to key structures, such as the central trachea and bronchi, the esophagus, the pulmonary artery, and the spinal cord. When the tolerance dose is exceeded in these organs, there is the risk of massive hemorrhage because of ulcers in the trachea or bronchi and peripheral bronchial occlusion [16,17]. However, there is a report of no complications with standard high-dose irradiation of 10-15 Gy as far as keeping normal tissue dose constraints [20]. Clinical trials are underway with the aim of finding optimal radiation doses.
In advanced-stage lung cancer, the irradiation volume increases, meaning that it is difficult to apply SBRT technology in its current form. However, after 60-70 Gy of 3D irradiation, it may be possible to conduct additional SBRT limited to the remaining tumor. Factors that influence additional radiation exposure include volume effect, fractionation effect, and total treatment duration. Total radiation dose distribution, including these biological factors, needs to be introduced into treatment planning in the future.
Future planning of 4D treatment should take into consideration time factors in existing geometric plans of 3D treatment. For example, even if the same dose of radiation is administered, the therapeutic effects differ greatly depending on the fractionated radiation dose and treatment duration. Furthermore, the final treatment plan may differ depending on the sensitivity to the initial irradiation. Ideally, treatment planning would take place before each irradiation. Gating and tracking are referred to as 4D treatment, but a new 4D treatment plan, including 4D CT, is expected in the future.
Current advances in mechanical engineering are outstanding. The IRGT devices are new image capture devices introduced into the irradiation room that reflect images captured before and after treatment. Several devices have been developed and employed clinically: the Hokkaido University realtime tumor-tracking radiotherapy irradiation device with on board imaging on the LINAC, a new irradiation device by Varian and Elekta, Cyberknife, Hyperknife (precession motion irradiation), Tomotherapy, and Vero. Development of these new irradiation devices may enable the future development of innovative irradiation techniques. Remarkable treatment outcomes have been reported in ion beam radiotherapy [21], and a comparative study against X-rays is awaited.
In Japan, SBRT is covered by public health insurance and is used for patients with primary lung cancer, metastatic lung cancer, primary liver cancer, metastatic liver cancer and arteriovenous malformation of the spinal cord.
There are treatment results for roughly 300 cases of liver tumors in Japan. However, radical resection, transcatheter arterial chemoembolization, local ethanol injection, and local ablation using radio frequency waves and microwaves are already conducted in everyday clinical practice. The treatment indications for SBRT in comparison to these established methods of treatment need better definition and guidelines. In the USA, an RTOG clinical trial is underway to determine the dose of radiation in SBRT suitable for primary liver cancer.
In addition to these diseases covered by health insurance, SBRT technology is extremely useful for any lesion limited to a local site. Therefore, renal cancers, adrenal gland tumors, paravertebral tumors, prostate cancer, and pancreatic cancer are being targeted [22].
References
1. Blomgren H, Lax I, Goranson H, Krapelien T, Nilsson B, Naslund I, et al. Radiosurgery for tumors in the body: clinical experience using a new method. J Radiosurg. 1998; 1:63–74.

2. Uematsu M, Shioda A, Tahara K, Fukui T, Yamamoto F, Tsumatori G, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer. 1998; 82:1062–1070. PMID: 9506350.
3. Onishi H, Kuriyama K, Komiyama T, Tanaka S, Sano N, Aikawa Y, et al. A new irradiation system for lung cancer combining linear accelerator, computed tomography, patient self-breath-holding, and patient-directed beam-control without respiratory monitoring devices. Int J Radiat Oncol Biol Phys. 2003; 56:14–20. PMID: 12694819.

4. Negoro Y, Nagata Y, Aoki T, Mizowaki T, Araki N, Takayama K, et al. The effectiveness of an immobilization device in conformal radiotherapy for lung tumor: reduction of respiratory tumor movement and evaluation of the daily setup accuracy. Int J Radiat Oncol Biol Phys. 2001; 50:889–898. PMID: 11429216.

5. Uematsu M, Shioda A, Suda A, Fukui T, Ozeki Y, Hama Y, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys. 2001; 51:666–670. PMID: 11597807.

6. Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005; 63:1427–1431. PMID: 16169670.

7. Onimaru R, Shirato H, Shimizu S, Kitamura K, Xu B, Fukumoto S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003; 56:126–135. PMID: 12694831.

8. Arimoto T, Usubuchi H, Matsuzawa T. Small volume multiple non-coplanar arc radiotherapy for tumors of the lund, head and neck and the abdominopelvic region. In : Lemke HU, editor. CAR'98 Computer assisted radiology and surgery. Tokyo: Elsevier;1998. p. 257–261.
9. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010; 303:1070–1076. PMID: 20233825.

10. Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys. 2004; 60:186–196. PMID: 15337555.

11. Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006; 66:117–125. PMID: 16765528.

12. Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009; 27:3290–3296. PMID: 19414667.

13. Fowler JF. Sensitivity analysis of parameters in linear-quadratic radiobiologic modeling. Int J Radiat Oncol Biol Phys. 2009; 73:1532–1537. PMID: 19306749.

14. Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004; 101:1623–1631. PMID: 15378503.
15. Takeda A, Enomoto T, Sanuki N, Nakajima T, Takeda T, Sayama K, et al. Acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by hypofractionated stereotactic body radiotherapy in a patient with primary lung cancer and slightly focal honeycombing. Radiat Med. 2008; 26:504–507. PMID: 18975053.

16. Nagata Y, Hiraoka M, Mizowaki T, Narita Y, Matsuo Y, Norihisa Y, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D Conformal External Beam Radiother-apy Group. Int J Radiat Oncol Biol Phys. 2009; 75:343–347. PMID: 19735861.

17. Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med. 2012; 366:2327–2329. PMID: 22694017.

18. Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006; 24:4833–4839. PMID: 17050868.

19. Inoue T, Shimizu S, Onimaru R, Takeda A, Onishi H, Nagata Y, et al. Clinical outcomes of stereotactic body radiotherapy for small lung lesions clinically diagnosed as primary lung cancer on radiologic examination. Int J Radiat Oncol Biol Phys. 2009; 75:683–687. PMID: 19231107.

20. Chang JY, Balter PA, Dong L, Yang Q, Liao Z, Jeter M, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008; 72:967–971. PMID: 18954709.

21. Miyamoto T, Baba M, Yamamoto N, Koto M, Sugawara T, Yashiro T, et al. Curative treatment of Stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007; 67:750–758. PMID: 17293232.

22. Nagata Y, Wulf J, Lax I, Timmerman R, Zimmermann F, Stojkovski I, et al. Stereotactic radiotherapy of primary lung cancer and other targets: results of consultant meeting of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys. 2011; 79:660–669. PMID: 21281896.

Fig. 1
A case of stereotactic body radiation therapy (SBRT) for early stage lung cancer (T1N0M0). SBRT is conducted on the left lung cancer by focusing radiation from six directions. Axial view, 3D image of radiation dose distribution.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download