Introduction
Hypercalcemia is a life threatening complication of malignant disease, and has been reported to occur in approximately 20% to 30% of patients with malignancies at some time during the course of their disease [
1]. Although hypercalcemia associated with cancer can be caused by various mechanisms, humoral hypercalcemia of malignancy (HHM) is the most common cause, accounting for 80% of occurrences. Parathyroid hormone-related peptide (PTH-rP) has been identified as a mediator of HHM [
2].
Cholangiocarcinoma (CC) is a relatively uncommon tumor, accounting for 10% to 15% of hepatobiliary malignancies and 3% of gastrointestinal tract cancers. The prognosis for advanced cholangiocellular carcinoma is very poor. In a recent large randomized controlled phase III study, gemcitabine plus cisplatin combination chemotherapy was beneficial in terms of progression-free survival and overall survival, as compared with gemcitabine monotherapy [
3], and has become the standard treatment for advanced CC; however, the median overall survival with combination chemotherapy arm is still less than one year.
HHM, which has rarely been reported in patients with CC, represents a marker of poor prognosis of the disease. In this case report, we present two cases of CC, showing the signs, symptoms, laboratory findings, and disease course consistent with HHM.
Go to :

Case Report
1. Case 1
A 63-year-old male patient was referred to our hospital for evaluation of an intrahepatic mass discovered during a routine health checkup in August 2010. He had no past medical history. Except for corrected serum calcium level of 12.1 mg/dL (normal range, 8.5 to 10.5 mg/dL), the blood test at initial presentation was not remarkable. His electrocardiogram showed normal sinus rhythm and there were no other hypercalcemia-associated symptoms or signs. An abdominopelvic computed tomography (CT) scan showed a large mass measuring 13 cm in size, suggesting CC with intrahepatic metastases and invasion of the left portal vein and hepatic vein (
Fig. 1A). Sono-guided liver biopsy was performed and the pathologic diagnosis showed poorly differentiated carcinoma, highly suggestive of CC. Because there was no distant metastasis on the positron emission tomography CT scan, we made a diagnosis of locally advanced intrahepatic CC. Given the tumor's unresectable status and the patient's good performance, we decided to treat him with radiotherapy concurrent with capecitabine 1,000 mg/m
2 twice daily on days 1-14 and cisplatin 60 mg/m
2 day once every three weeks. The first two cycles of chemotherapy were administered with radiotherapy, a total dose of 45 Gy at the main mass in the liver.
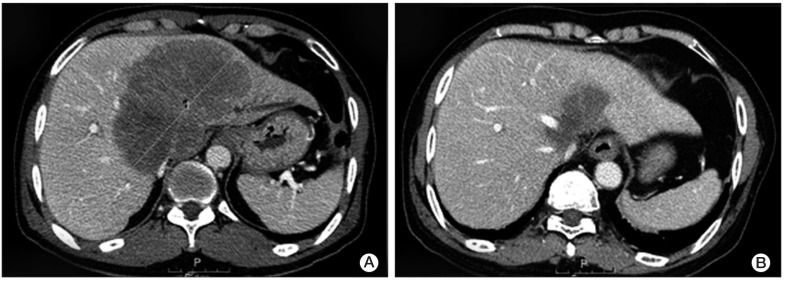 | Fig. 1(A) Computed tomography (CT) scan at initial diagnosis showing intrahepatic metastases measuring 13 cm and invasion of the Lt. portal vein and hepatic vein. (B) Six months later, follow up CT scan after radiation therapy concurrent with capecitabine plus cisplatin showed marked improvement of tumor status. 
|
Follow-up CT scan after concurrent chemoradiotherapy (CCRT) showed marked improvement in the patient's tumor status (
Fig. 1B). In parallel with the improvement of tumor status, serum calcium level showed a decrease to the normal range (
Fig. 2). We continued to administer the same treatment regimen, and the response was maintained for nine months, until May 2011, when a follow-up CT scan showed liver metastasis progression. Carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) level were elevated to 48.17 U/mL (normal range, 0 to 5 U/mL) and 97.0 ng/mL (normal range, 0 to 37 ng/mL), respectively. At the same time, his corrected serum calcium level increased again to 11.1 mg/dL. The suppressed PTH level (5.1 pg/mL; normal range, 15 to 65 pg/mL) with elevated PTH-rp level (6.7 pmol/L; normal range, 0 to 1.1 pmol/L) confirmed the diagnosis of recurrent HHM. We treated the patient with intravenous hydration and pamidronate. Hypercalcemia was resolved in five days and the patient was performing well without any signs or symptoms related to hypercalcemia, therefore, he was treated with second-line chemotherapy with gemcitabine.
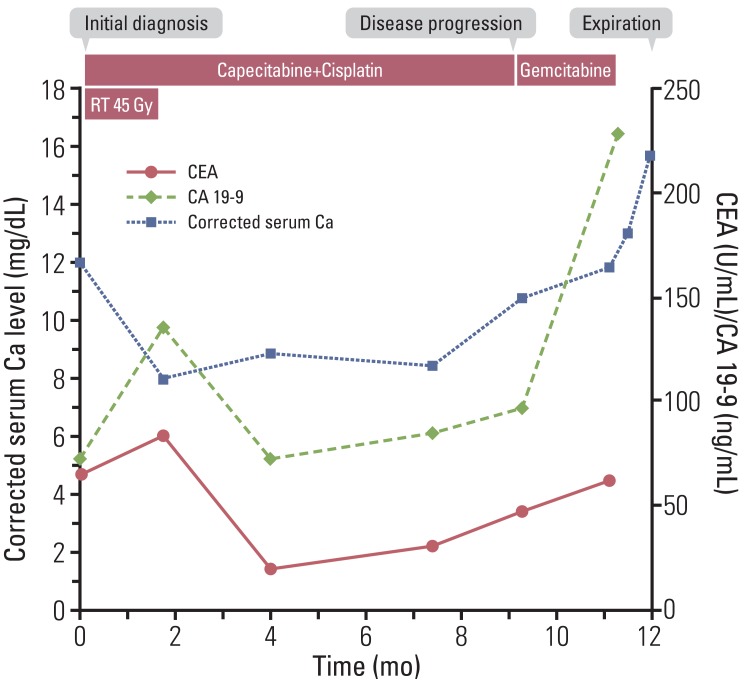 | Fig. 2Patient progress, including changes in corrected serum calcium level and tumor marker. CEA, carcinoembryonic antigen; CA 19-9, cancer antigen 19-9. 
|
After two cycles of gemcitabine, a follow-up CT scan showed liver mass progression and newly developed hematogenous lung metastasis. The corrected serum calcium level was elevated even higher (11.8 mg/dL). Although hypercalcemia was normalized after medical treatment, his poor performance hindered him from receiving additional chemotherapy. Instead, he was treated with the best supportive care. When he visited the outpatient clinic three weeks later, he was in a state of somnolence and oral intake was not possible due to severe nausea and vomiting. His corrected serumcalcium level rose upto 15.3 mg/dL.
Eventually, the patient died on 10 August 2011, almost one year after initial diagnosis of CC with HHM.
2. Case 2
A 68-year-old male patient presented with weight loss of 6 kg over two months and abdominal pain for one week. He had no past medical history. Ultrasonography performed at the local hospital showed a mass lesion in the liver, and he was referred to our hospital. An abdominopelvic CT scan showed CC with innumerable liver to liver metastases and node metastases. Sono-guided liver biopsy was performed and the pathologic diagnosis was adenocarcinoma, either primary or metastatic. Because no primary mass was found by esophagogastroduodenoscopy and colonoscopy, we made a diagnosis of CC with liver metastasis and began treatment with palliative chemotherapy, gemcitabine plus cisplatin. His tumor was stable until 28 June 2011, when he visited the outpatient clinic for the eighth cycle of gemcitabine and cisplatin approximately six months after his initial diagnosis.
At that time, his blood test was remarkable for a corrected serum calcium level of 11.8 mg/dL. Otherwise, there were no symptoms or signs related to hypercalcemia and he was in good performance status. After receiving treatment with intravenous hydration and pamidronate, he received the 8th cycle of chemotherapy as scheduled. We scheduled an appointment for another visit to recheck his condition as well as his serum calcium level. When he returned to the outpatient clinic one week later, his mental status was somewhat somnolent. A blood test showed an aggravated corrected serum calcium level of 14.1 mg/dL and decreased liver function; aspartate aminotransferase was three times the normal level (102 IU/mL; normal range, 13 to 34 IU/mL), alkaline phosphatase was 340 U/L (normal range, 38 to 115 U/L), and albumin was decreased to 2.8 mg/dL (normal range, 3.3 to 5.3 mg/dL). Other blood tests were not remarkable. CT scan revealed aggravation of the main mass and a metastatic lesion in the liver, and newly developed ascites. CEA and CA 19-9 levels were elevated to 981.32 ng/mL and 7,450 U/mL, respectively. As the PTH level was suppressed to 4.8 pg/mL and PTH-rP level was increased to 8.3 pmol/L, we were able to make a diagnosis of HHM. He was hospitalized and treated with intravenous hydration and pamidronate. After admission, his hypercalcemia was corrected to a certain degree, but never returned to normal range. Despite improvement in his mental status, his performance status did not recover from Eastern Cooperative Oncology Group 3. Hence we discontinued chemotherapy and treated him with the best supportive care until he expired on 24 July 2011, only approximately one month after diagnosis of HHM.
Go to :

Discussion
HHM is usually found in patients with squamous cell carcinoma as of the head and neck, esophagus, and lung. Other tumors commonly associated with HHM include breast, renal, bladder, and ovarian cancers, human T-cell lymphotropic virus-1 lymphoma, and some endocrine tumors.
CC is rarely associated with HHM. In an early retrospective analysis conducted in 1982, when measurement of PTH-rP was not available, Oldenburg et al. [
4] reported hypercalcemia of unknown origin in seven out of 40 patients (17.5%) with CC. Among them, immunoreactive parathyroid hormone (iPTH) concentration levels were measured in only two patients. Both patients had normal or low PTH levels, and the authors concluded their works by suggesting that secretion of iPTH-like peptide is the possible mechanism for their findings [
4].
Since the introduction of PTH-rP to clinical practice in 1987, only 10 cases of HHM associated with CC have been reported in the PubMed/Medline database [
5]. Most previously reported cases were from Japan and showed male predominance and predilection for the right lobe. In most cases, development of HHM occurred just weeks or months before the patients expired, representing a heavy disease burden and a dismal prognosis.
Although there are many factors known to be associated with the pathogenesis of HHM, PTH-rP is believed to be the major mediator among them; its level is elevated in 80% of patients with HHM [
2]. PTH-rP causes hypercalcemia through enhancement of tubular reabsorption of calcium and stimulation of osteoclastic bone resorption [
6]. Therefore, HHM treatment should be directed toward enhancement of urinary calcium excretion by vigorous hydration and intravenous loop diuretics, and blocking bone reabsorption with calcitonin and biphosphonates [
7].
To the best of my knowledge, these are the first two case reports in Korean PubMed/Medline and they are notable in several points.
First, hypercalcemia was detected upon initial manifestation of the tumor in our first case. Although we did not measure PTH-rP at that time, the absence of bone metastasis and the finding of hypercalcemia normalization and aggravation in accordance with the disease course lead us to postulate that this patient had an association with HHM from the time of diagnosis. This type of presentation is unusual and has only been reported in two cases [
5,
8].
Second, given the usual life expectancy of several weeks to months after detection of HHM in CC patients, the patient reported in the first case showed a remarkably long survival time, almost one year. Only two case studies showing longer survival time than one year after initial diagnosis of HHM with CC have been reported [
9,
10]. In one case report, the patient underwent right portal vein embolization and chemoembolization followed by extended right hepatectomy, and survived three and one half years [
10]. In the other case report, the patient received CCRT with gemcitabine and TS-1, and survived 14 months [
9]. The patient described in our report received treatment with CCRT with oral capecitabine. Although there are some studies suggesting that chemoradiation in advanced CC can prolong survival time, its role has focused mainly on symptom palliation due to local tumor effects [
11,
12]. Until now, 5-fluorouracil (5-FU) has been the most thoroughly investigated chemotherapeutic agent in CCRT for treatment of biliary tract cancers, however, because some studies have reported that capecitabine can replace 5-FU without severe toxicity, we chose oral capecitabine [
13]. The survival prolongation observed in these patients suggests that poor prognosis in HHM associated with CC can be partly overcome when the tumor is controlled by aggressive treatment, such as surgery or CCRT. In addition, a strong correlation was observed between tumor response to CCRT and the level of hypercalcemia.
Third, consistent with the previous reports, our reports showed overall dismal prognosis of HHM associated with CC. The second patient expired only four weeks after the diagnosis of HHM. Although the first patient survived longer than one year after the first event of HHM, there was only one month until his demise since recurrence of HHM. Both cases indicated that HHM is refractory to medical treatment and led to a fatal outcome when it developed in a patient whose tumor showed progression upon active anticancer therapy.
In conclusion, we herein report on two cases of humoral hypercalcemia of malignancy associated with CC. Although the condition's dismal prognosis can be partly overcome by more proactive treatment, its manifestation implicates massive overall disease burden and an adverse outcome when the tumor itself is not controlled.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download