Introduction
Materials and Methods
1. Study design
2. Treatment
3. Response, toxicity, recurrence and survival
4. Statistical analysis
Results
1. Patient characteristics
Table 1
| Characteristics | Group 1 (n=19) | Group 2 (n=18) |
|---|---|---|
| Median age (range, yr) | 48 (24-72) | 52 (37-74) |
| Menopause | 8 (42.1) | 13 (72.2) |
| ECOG performance status | ||
| 0 | 17 (89.5) | 16 (88.9) |
| 1 | 2 (10.5) | 2 (11.1) |
| FIGO stage | ||
| IB1 | 13 (68.4) | - |
| IB2 | 2 (10.5) | - |
| IIA | 4 (21.1) | - |
| IIB | - | 12 (66.7) |
| IIIA | - | 2 (11.1) |
| IVA | - | 4 (22.2) |
| Median tumor size (range, cm) | 4.0 (2.1-9.0) | 4.2 (2.5-8.9) |
| Histology | ||
| Squamous carcinoma | 15 (78.9) | 17 (94.4) |
| Adenocarcinoma | 2 (10.5) | 1 (5.6) |
| Adenosquamous carcinoma | 1 (5.3) | 0 (0) |
| Mixed carcinoma | 1 (5.3) | 0 (0) |
| Lymph-vascular space invasion | ||
| No | 14 (73.7) | - |
| Yes | 5 (26.3) | - |
| Stromal invasion | ||
| <1/2 | 17 (89.5) | - |
| ≥1/2 | 2 (10.5) | - |
| Parametrial invasion | ||
| No | 13 (68.4) | - |
| Yes | 6 (31.6) | - |
| Lymph node metastasis | ||
| No | 16 (84.2) | 17 (94.4)a) |
| Yes | 3 (15.8) | 1 (5.6)a) |
| Positive resection margin | ||
| No | 19 (100) | - |
| Yes | 0 (0) | - |
2. Consolidation chemotherapy after post-surgery adjuvant CCR
1) Toxicity
Table 2
2) Recurrence and survival
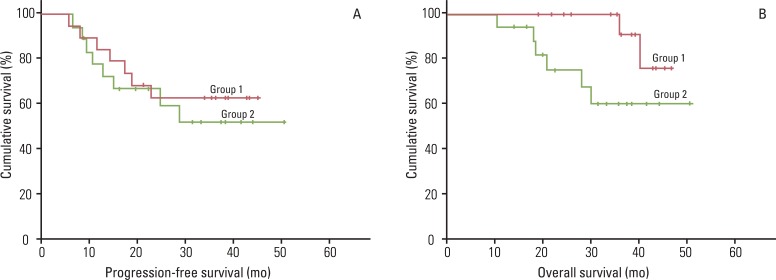 | Fig. 1Efficacy of paclitaxel and carboplatin as consolidation chemotherapy after concurrent chemoradiation (CCR) in patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB1-IIA (group 1) and stage IIB-IVA (group 2) cervical cancer; (A) progression-free survival and (B) overall survival curves for 19 patients who underwent primary surgery followed by three cycles of consolidation chemotheray after CCR (group 1) and 18 patients who received primary CCR followed by three cycles of consolidation chemotherapy (group 2). |
3. Consolidation chemotherapy after primary CCR
1) Toxicity
2) Response, recurrence and survival
Discussion
Table 3
| Study | Study design | No. of patients | FIGO stage | Histology | Primary treatment | RT | Concurrent chemotherapy | Consolidation chemotherapy | CR (%) | Grade 3-4 common toxicity (%) | Survival (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peters et al. [18] | Phase III | 127 | IA-IIA |
SC AC ASC |
Surgery | EBRT | 2 cycles of F (1 g/m2)/P (70 mg/m2) every 3 wk | 2 cycles of F (1 g/m2)/P(70 mg/m2) every 3 wk | - |
Neutropenia(9.0)a) Diarrhea (3.3)a) |
81 (4 yr OS) 80 (4 yr PFS) |
| Vrdoljak et al. [7] | Phase II | 44 | IB2-IVA | SC | CCR | EBRT+LDR | 2 cycles of I (2 g/m2)/P (75 mg/m2) every 3 wk | 2 cycles of I (2 g/m2)/P (75 mg/m2) every 3 wk | 100 | Leukopenia (36)a) | 84 (PFS) |
| Nausea (8.0)a) | 91 (OS) | ||||||||||
| Vomiting (8.0)a) | |||||||||||
| Proctitis (6.8)a) | |||||||||||
| Cystitis (4.5)a) | |||||||||||
| R-V fistula (4.5)a) | |||||||||||
| Ureteral obstruction (4.5)a) | |||||||||||
| V-V fistula (2.3)a) | |||||||||||
| Chung et al. [8] | Phase I/II | 63 | IIB-IVA | SC | CCR | EBRT+HDR | 2 cycles of P (50-80 mg/m2) every 4 wk | 2 cycles of F (600-800 mg/m2)/P (60-80 mg/m2) every 4 wk | - | Proctitis (3.0)a) | 81-86 (3 yr PFS) |
| AC | Cystitis (2.0)a) | 81 (3 yr OS) | |||||||||
| ASC | |||||||||||
| Choi et al. [9] | Phase II | 32 | IB2-IVA | SC | CCR | EBRT+HDR | 3 cycles of F (1 g/m2)/P (60 mg/m2) every 3 wk | 3 cycles of F (1 g/m2)/P (60 mg/m2) every 3 wk | 87 | Neutropenia (7.5)b) | 83 (3 yr PFS) |
| AC | Anemia (7.0)b) | 91 (3 yr OS) | |||||||||
| ASC | Nausea/Vomiting (7.0)b) | ||||||||||
| Zhang et al. [19] | Phase II | 34 | IIB-IIIB | SC | CCR | EBRT+HDR | 6 cycles of T (35 mg/m2)/N (20 mg/m2) every 1 wk | 4 cycles of T (135 mg/m2)/N (60 mg/m2) every 3 wk | 88 | Leukopenia (10.9)b) | 82 (2 yr PFS) |
| Neutropenia (9.2)b) | 95 (2 yr OS) | ||||||||||
| Nausea/Vomiting (2.0)b) | |||||||||||
| Proctitis (5.9)b) | |||||||||||
| Cystitis (3.0)b) | |||||||||||
| Kim et al.c) | Phase II | 10 | IB1-IIA | Surgery | EBRT+LDR | 3 cycles of T (135 mg/m2) /C (AUC 5.0) every 3 wk | 3 cycles of T (175 mg/m2)/C (AUC 5.0) every 3 wk | - | Leukopenia (10.5)b) | 62.7 ( 3yr PFS) | |
| Neutropenia (7.0)b) | 90.9 (3 yr OS) | ||||||||||
| SC | Diarrhea (1.8)b) | ||||||||||
| AC | Cystitis (5.3)b) | ||||||||||
| 14 | IB1-IIA | ASC | CCR | 77.8 | Leukopenia (13.0)b) | 51.9 (3 yr PFS) | |||||
| Mixed | Neutropenia (14.8)b) | 60 (3 yr OS) | |||||||||
| Febrile illness (1.9)b) | |||||||||||
| Proctitis (5.6)b) |
CCR, concurrent chemoradiation; FIGO, International Federation of Gynecology and Obstetrics; RT, radiation therapy; CR, complete response; SC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous carcinoma; EBRT, external beam radiation therapy; F, 5-fluorouracil; P, cisplatin; PFS, progression-free survival; OS, overall survival; I, ifosfamide; LDR, low dose rate intracavitary irradiation; R-V, rectovaginal; V-V, vesicovaginal; HDR, high dose rate intracavitary brachytherapy; T, paclitaxel; N, nedaplatin; C, carboplatin. a)Number of events per patients, b)Number of events per cycles of chemotherapy, c)The current study.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download