Abstract
Coombs' negative autoimmune hemolytic anemia (AIHA) is a rare disease which shares similar clinical and hematological features with Coombs' positive AIHA, but its exact frequency remains unknown. There have been few reports of idiopathic thrombocytopenic purpura (ITP) and Coombs' negative AIHA associated with other lymphoproliferative disorders (LPDs). Since there is a well known association between LPDs and autoimmune phenomena, it is important to investigate the possibility of an underlying malignancy. We report a case of ITP and Coombs' negative AIHA associated with diffuse large B-cell lymphoma.
There is a well-described association between lymphoproliferative disorders (LPDs) and autoimmune hemolytic anemia and thrombocytopenia. An 8.7-year, retrospective study of 626 non-Hodgkin's lymphoma (NHL) patients reported 16 patients (2.6%) with autoimmune hemolytic anemia (AIHA), 11 patients (1.8%) with thrombocytopenia of presumed autoimmune origin, and 3 patients (0.5%) with Evans' syndrome [1]. Unfortunately, there have not been enough studies to evaluate the associations between each of the types of LPDs, AIHA, or idiopathic thrombocytopenic purpura (ITP). To our knowledge, this is the first Korean report to describe an adult with Coombs' negative hemolytic anemia and ITP who was later diagnosed with diffuse large B-cell lymphoma. Coombs' negative hemolytic anemia and ITP occurring together with NHL is very rarely reported. Since there is a well described association of autoimmune phenomenon and LPDs, the importance of recognizing the possibility of an underlying malignancy with AIHA should always be considered.
A 51-year-old, male patient was admitted to our hospital in May 2005, suffering with epistaxis. He had no history of previous medical disorders, and had never had a blood transfusion. He had not taken any kind of medication prior to manifestation of symptoms and was neither an alcoholic nor a heavy drinker. The patient's blood pressure was 126/77 mm Hg, his pulse rate was 87 beats/min, his respiratory rate was 22 breaths/min, and his body temperature was 36℃.His initial hemoglobin level was 14 g/dL, his hematocrit was 43.8% and his white blood cell count (WBC) was 4,400×103/µL. His platelet count was observed to be low, at 16×103/µL. Hepatomegaly and splenomegaly were both not observed during physical examination. Mild hepatosplenomegaly was seen in his liver scan accompanied by reticuloendothelial system dysfunction. Increased numbers of megakaryocytes were seen in the result of the patient's bone marrow aspiration (Fig. 1). He was diagnosed with ITP, and the patient started treatment with high-dose oral prednisolone at 1 mg/kg/day for 4 weeks, and his platelet count level subsequently rose to 204×103/µL. He was discharged and visited our outpatient clinic routinely for the next 6 months, continuing with low-dose oral prednisolone tapered to 5 mg.
Seven months later, during a routine visit to the clinic he complained that his face had developed a yellowish color. Upon physical examination, icteric sclera and anemic conjunctiva were revealed. Upon laboratory examination, his hemoglobin and hematocrit levels were observed to be 7.0 g/dL and 23.2%, respectively. His WBC and platelet counts were found to be 4,400/µL and 241×103/µL, respectively. His corrected reticulocyte count was 8.3% and reticulocyte production index (RPI) was 4.1. His total and direct bilirubin concentrations were observed to be 4.5 mg/dL and 0.6 mg/dL respectively. His lactate dehydrogenase concentration was high, at 937 IU/L, and his serum haptoglobin had decreased to 10 mg/dL. Acidified sucrose lysis (Ham's test), antinuclear antibody, and anti-dsDNA antibody tests all produced negative results. Both direct and indirect Coombs' tests both produced negative results. Flow cytometry for red blood cell CD55 and CD59 were 99.8% (neutrophil 99.1%) and 99.9% (neutrophil 99.9%), respectively. Peripheral blood smear revealed macrocytic normochromic anemia (Fig. 2A). A bone marrow biopsy was repeated, which revealed normocellular marrow with increased erythroid and megakaryopoiesis (Fig. 2B). Based on these findings, we concluded that the patient suffered from ITP and Coombs' negative AIHA. Since there is a well known association between LPDs and AIHA, an F-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) scan was conducted to investigate possible occurrence of LPDs, however, there was no sign of malignancy (Fig. 3A). He was treated with a higher dosage of oral prednisolone of one [1] mg/kg/day for 5 weeks. We attempted to taper the prednisolone dosage, but his hemoglobin level fluctuated in proportion to the dose. We determined that the patient was developing a steroid dependency and a resulting laparoscopic splenectomy was performed in November 2006. After the operation, his hemoglobin level and platelet count slowly increased and reached normal range. His hemoglobin level was 13.8 g/dL and his platelet count was 301×103/µL. We stopped the steroid treatment.
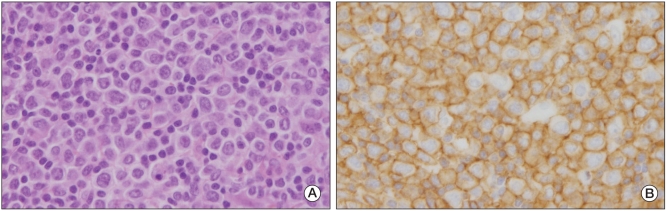
During a routine clinic visit four months later, the patient complained of a yellowish face color again. He also had a newly developed, palpable lymph node on the right side of his neck. His hemoglobin count had decreased to 7.4 g/dL and his platelet count had increased to 529×103/µL. His corrected reticulocyte count was 6.6% and his RPI was 3.3. His lactate dehydrogenase concentration had increased to 1,088 IU/L and his serum haptoglobin had fallen to 21.1 mg/dL. Direct and indirect Coombs' test results were again, both negative. A peripheral blood smear revealed macrocytic and normochromic erythrocytes with mild anisocytosis and poikilocytosis. His chest radiograph, taken on admission, revealed a right hilar prominence which was not apparent in the chest radiograph taken 4 months before. A chest CT scan and F-18 FDG PET/CT scan confirmed enlarged lymph nodes in the right hilum, mediastinum, and right neck (Fig. 3B). A biopsy was performed using the enlarged lymph node on the right side of his neck, revealing diffuse proliferation of medium to large atypical lymphoid cells (Fig. 4). The patient was diagnosed with diffuse large B-cell lymphoma. Bone marrow aspiration and biopsy revealed hypercellular marrow, but no evidence of malignant lymphoma. He was treated with 8 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). After the 8 cycles of chemotherapy, complete remission was achieved. The patient is still visiting our outpatient clinic regularly. Full blood count, including reticulocytes, and blood films have all remained within normal limits.
Evans' syndrome is an uncommon disorder in which autoimmune hemolytic anemia and idiopathic thrombocytopenic purpura coexist without a known underlying etiology [1-3]. Usually, Evans' syndrome is found with Coombs' positive AIHA and ITP, but a few cases with Coombs' negative AIHA and ITP have been reported as well. The corner stone of AIHA diagnosis is a positive direct Coombs' test [4], but in rare cases, Coombs' negative AIHA cases are also reported and the exact frequency is unknown [4,5]. In a previous report covering a series of 79 cases, 6 patients were found to be Coombs' negative by the direct antiglobulin test (DAT) [4]. Evans' and Weiser, described identification of 4 patients with negative Coombs' test results among a total of 41 patients confirmed with AIHA [5]. Negative Coombs' test results could be due to low numbers of antibodies on red cell membranes or the low sensitivity of the conventional tube method used for performing DAT [4,5]. We repeated both direct and indirect Coombs' tests for our patient twice, and both tests produced negative results. In our laboratory, the conventional tube method was used for performing both the direct and indirect Coombs' tests. The sensitivity of the Coombs' test can be improved by using an AutoAnalyzer with polyvinylpyrrolidone [5].
There is a well-described association between LPDs and AIHA [1,6-11]. In a previous study, it was estimated that 4.3% of patient with chronic lymphocytic leukemia (CLL) developed AIHA [9,11]. But unfortunately, occurrence of AIHA in other types of LPDs such as NHLs has not been investigated in a large scale study [9]. LPDs associated with Evans' syndrome have rarely been described [11,12]. In a 7-year, retrospective study of 107 AIHA patients, 19 (18%) later developed malignant LPDs [9]. Among the various types of malignant LPDs, CLL is most frequently associated with AIHA [11]. Other malignant LPDs reported to be associated with Evans' syndrome include Hodgkin's disease, splenic marginal zone lymphoma, gastric plasmacytoma, hairy cell leukemia, and diffuse large B-cell lymphoma [6,7,12,13]. To our knowledge, there are limited numbers of reported cases of lymphoma associated with Coombs' negative AIHA and ITP [14]. The importance of regular follow-ups, including laboratory examinations to investigate the possibility of hidden malignancy, is noted in such patients. Because its presentation is quite variable, Coombs' negative AIHA and ITP should be identified as soon as possible such that an early diagnoses can be made and appropriate therapy provided [4]. Although steroids and immunoglobulins remain the mainstay of treatment for this condition, failure of patient response is common, and prognosis remains uncertain [2]. In its more severe form, overwhelming hemolysis is associated with hemoglobinemia, hemoglobinuria and shock, which can be fatal unless aggressively treated [4]. Within this context, we report a case of diffuse large B-cell lymphoma, diagnosed during the regular follow up period after complete remission of Coombs' negative AIHA and ITP. In our view, periodic clinical and laboratory evaluation is essential in such patients because it is difficult to rule out the possibility of hidden malignancies. More large scale studies on the associations between lymphoma and Coombs' negative AIHA and ITP needed to be conducted.
References
1. Grønbaek K, D'Amore F, Schmidt K. Autoimmune phenomena in non-Hodgkin's lymphoma. Leuk Lymphoma. 1995; 18:311–316. PMID: 8535198.
2. Molla MA, Biswas AC, Tohany AM, AI Moslem K. Evans's syndrome: a case report and review of the literature. Ann Saudi Med. 2001; 21:255–256. PMID: 17264570.

3. Ganly PS, Laffan MA, Owen I, Hows JM. Auto-anti-Jka in Evans' syndrome with negative direct antiglobulin test. Br J Haematol. 1988; 69:537–539. PMID: 3408690.
4. Gupta R, Singh DK, Singh S, Singh T. Coomb's negative autoimmune hemolytic anemia: a diagnostic dilemma for the hematologist. Indian J Pathol Microbiol. 2008; 51:571–572. PMID: 19008606.

5. Gilliland BC. Coombs: negative immune hemolytic anemia. Semin Hematol. 1976; 13:267–275. PMID: 1006330.
6. Keung YK, Cobos E, Bolanos-Meade J, Issarachai S, Brideau A, Morgan D. Evans syndrome after autologous bone marrow transplant for recurrent Hodgkin's disease. Bone Marrow Transplant. 1997; 20:1099–1101. PMID: 9466285.

7. García-Muñoz R, Rodriguez-Otero P, Pegenaute C, Merino J, Jakes-Okampo J, Llorente L, et al. Splenic marginal zone lymphoma with Evans' syndrome, autoimmunity, and peripheral gamma/delta T cells. Ann Hematol. 2009; 88:177–178. PMID: 18665362.

8. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002; 69:258–271. PMID: 11921020.

9. Sallah S, Wan JY, Hanrahan LR. Future development of lymphoproliferative disorders in patients with autoimmune hemolytic anemia. Clin Cancer Res. 2001; 7:791–794. PMID: 11309323.
10. Váróczy L, Gergely L, Zeher M, Szegedi G, Illés A. Malignant lymphoma-associated autoimmune diseases: a descriptive epidemiological study. Rheumatol Int. 2002; 22:233–237. PMID: 12426661.
11. Mauro FR, Foa R, Cerretti R, Giannarelli D, Coluzzi S, Mandelli F, et al. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood. 2000; 95:2786–2792. PMID: 10779422.

12. Yonekura S, Nagao T, Arimori S, Miyaji M, Ogoshi K, Tsutsumi Y. Evans' syndrome associated with gastric plasmacytoma: Case report and a review of the literature. Jpn J Med. 1990; 29:512–515. PMID: 2089175.
13. Hauswirth AW, Skrabs C, Schützinger C, Gaiger A, Lechner K, Jäger U. Autoimmune hemolytic anemias, Evans' syndromes, and pure red cell aplasia in non-Hodgkin lymphomas. Leuk Lymphoma. 2007; 48:1139–1149. PMID: 17577777.

14. Kondo H, Oyamada T, Mori A, Sumi H, Kurosu K, Kajii E, et al. Direct-antiglobulin-test-negative immune haemolytic anaemia and thrombocytopenia in a patient with Hodgkin's disease. Acta Haematol. 2001; 105:233–236. PMID: 11528097.

Fig. 1
Bone marrow aspiration showed normocellular marrow with increased immature megakaryocyte (H&E staining, ×200).

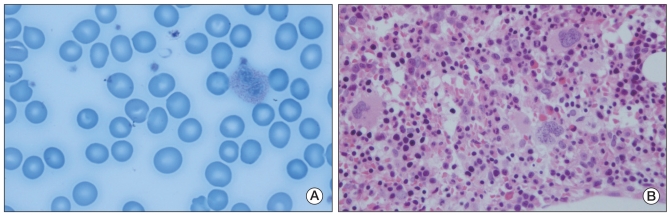
Fig. 2
(A) Peripheral blood smear revealed macrocytic normochromic anemia (H&E staining, ×1,000). (B) Bone marrow biopsy shows normocellular marrow with increased erythroid and megakaryopoiesis (H&E staining, ×400).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download