Abstract
Purpose
CD10, a membrane-bound zinc-dependent metallopeptidase, is normally expressed in many tissues. Accordingly, the derangement of CD10 expression may be related to development or progression in a variety of tumors. The aim of this study is to examine any association between CD10 expression and clinicopathological parameters in bladder transitional cell carcinomas (TCCs) and the relationship between expression of E-cadherin and CD10.
Materials and Methods
Immunohistochemical staining was performed for CD10 and E-cadherin in tissues of 94 TCCs and 10 non-neoplastic bladder mucosa.
Results
Positive immunoreactivity for CD10 was observed in non-neoplastic urothelium at a proportion of 80% and TCCs were observed at a rate of 23%. A positive rate of CD10 expression was observed in 10% of total cases of a low grade tumor and in 35% of those of a high grade tumor. It was also observed in 15% of pTa tumors, 13% of pT1 tumors, and 48% of pT2 tumors. In addition, CD10 expression showed reciprocal correlation with expression of membranous E-cadherin in tumors.
Matrix metallopeptidases (MMPs) have an important role in cancer progression through release of bioactive molecules that inhibit apoptosis and stimulate invasion, degradation of extracellular matrix components, promotion of angiogenesis, and modulation of immune response [1,2]. MMPs also induce mitotic abnormalities and genomic instability by cleavage of intracellular targets [3]. In addition, MMPs can also stimulate processes associated with epithelial-to-mesenchymal transition (EMT) through cleavage of E-cadherin and production of activated splice variant Rac1b, stimulating elevation of the levels of cellular reactive oxygen species, which in turn leads to an increase in expression of Snail [3,4]. CD10 (common acute lymphoblastic leukemia antigen) is a membrane-bound zinc-dependent metallopeptidase, which regulates the physiological action of various peptides by lowering their extracellular concentration available for receptor binding [5]. CD10 is normally expressed in many tissues, including epithelial cells of the kidney, breast, lung, intestine, and prostate; accordingly, derangement of CD10 expression has been associated with development or progression in a variety of tumors [5-7]. Conflicting results have been reported in bladder transitional cell carcinomas (TCCs), with CD10 downregulation in progressive tumors, or, inversely, its upregulation has been associated with invasion and metastasis [8-12].
Based on the fact that CD10 is a family of MMPs, the aim of this study is to examine any association between CD10 expression and clinicopathological parameters in bladder TCCs and the relationship between expression of E-cadherin and CD10.
We used 94 paraffin-embedded bladder TCCs and 10 normal bladder tissues obtained from the Department of Pathology at Dongguk University Gyeongju Hospital. Cancer tissues were obtained from transurethral resection of bladder TCCs. Normal bladder epithelial tissues were obtained from cases of chronic cystitis. Tumors were graded in accordance with the World Health Organization-International Society of Urological Pathology (WHO-ISUP) classification [13], and the pathological T stage (pT, depth of invasion) was also determined. Thirty nine tumors were stage pTa, 30 were pT1, and 25 were more or equal to pT2. Forty two patients had low grade tumors, while 52 patients had high grade tumors. The age distribution of patients was between 30 and 87 years old, and the male to female ratio was 6.2 : 1.
Disease recurrence was defined as any evidence of tumor in a retained bladder at least three months after treatment. Of the 68 available patients, 20 patients had recurrent tumors.
Urinary bladder sections of 4 µm thickness were made and were spread on poly-L-lysine coated slides. The paraffin sections were immersed in three changes of xylene and hydrated using a graded series of alcohol solutions. Antigen retrieval was performed routinely by immersing the sections in a 0.01 M citrate buffer (pH 6.0) in an autoclave for 15 minutes. Endogenous peroxidase activity was blocked with a 3% hydrogen peroxide for 15 minutes, followed by incubation of the sections with primary antibody for two hours at room temperature; the primary antibodies included mouse monoclonal anti-E-cadherin (1 : 1,000, Transduction Laboratories, Lexington, KY) and anti-CD10 (1 : 100, NCL-CD10-270, Novocastra Laboratories Ltd., Newcastle, UK). Immunohistochemical staining was performed using an EnVision kit (Dako, Santa Barbara, CA) and the color was developed with 3, 3'-diaminobenzidine tetrahydrochloride (Zymed Laboratories, Inc., South San Francisco, CA) as a chromogen. The sections were counterstained with Meyer's hematoxylin for three minutes and then mounted. Mouse IgG isotype, rather than the primary antibody, was used as a negative control. The immunoreactivity of membranous E-cadherin was evaluated based on the extensity, and our cases were classified into a normal group (stained tumor cells, >90%) and an abnormal group (stained tumor cells, 0-90%). The immunoreactivity of CD10 was also evaluated based on the extensity of tumor cells and non-neoplastic urothelial cells. Extensity was graded according to a 4-point scale based on the percentage of stained cells: 0 (stained cells, 0-10%), 1 (stained cells, 11-20%), 2 (stained cells, 21-50%), and 3 (stained cells, >50%). Our cases were divided into two groups: the negative group (0, 1) and the positive group (2, 3).
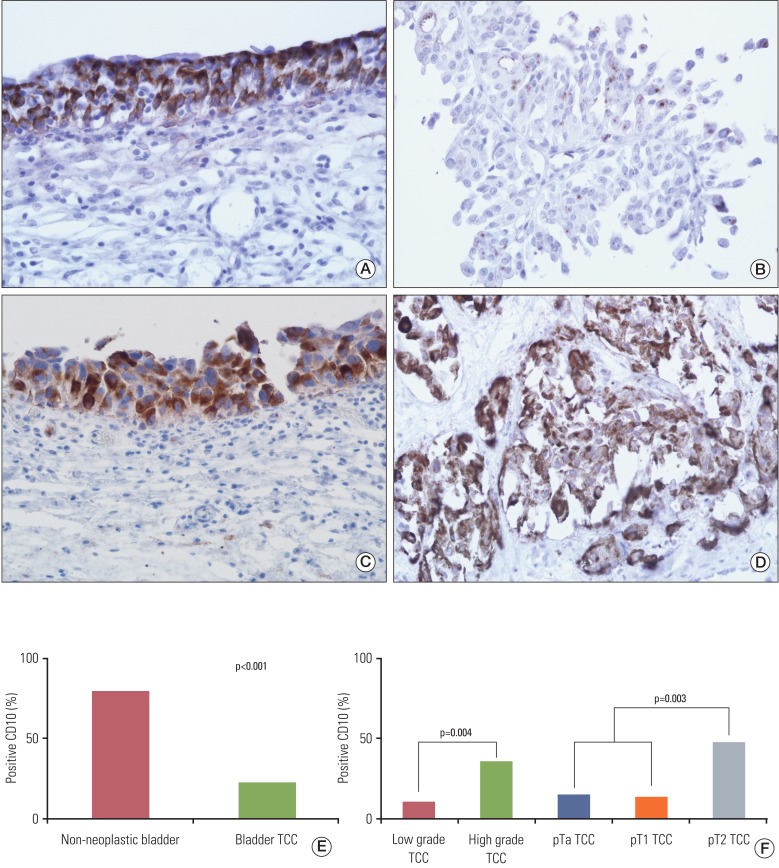
CD10 staining was predominantly cytoplasmic in tumor, non-neoplastic epithelial and stromal cells, however, membranous staining was also observed. It did not show significant correlation with gender and age (data not shown). Positive immunoreactivity for CD10 was observed in normal urothelium at a proportion of 80% (8/10) and TCCs were observed at a rate of 23% (22/94). CD10 expression was significantly higher in normal bladder mucosa, compared with TCCs (p<0.001) (Fig. 1).
We analyzed CD10 expression according to grade, depth of invasion, and status of tumor recurrence in TCCs. A positive rate of CD10 expression was observed in 10% (4/42) of total cases of a low grade tumor and in 35% (18/52) of those of a high grade tumor. In addition, it was also observed in 15% (6/39) of pTa tumors, 13% (4/30) of pT1 tumors, and 48% (12/25) of pT2 tumors. Eight pT2 tumors showed carcinoma in situ in an adjacent area, where strong expression of CD10 was observed in four cases (Fig. 1). In addition, it was observed in 19% (9/48) of tumors without recurrence and 15% (3/20) of tumors with recurrence. CD10 expression showed significant correlation with tumor grade (p=0.004) and tumor invasion (p=0.003), however, it did not show correlation with tumor recurrence (Fig. 1).
We analyzed E-cadherin expression according to grade and depth of invasion in TCCs (Fig. 2). Abnormal E-cadherin expression was observed in 5% (2/42) of total cases of a low grade tumor and in 54% (28/52) of those of a high grade tumor. In addition, it was observed in 13% (5/39) of pTa tumors, 33% (10/30) of pT1 tumors, and 60% (15/25) of pT2 tumors. Abnormal E-cadherin expression showed significant correlation with high grade and tumor invasion (p<0.001).
We analyzed the relationship between expression of CD10 and E-cadherin in TCCs. A degree of CD10 expression was observed in 37% (11/30) of tumors with abnormal E-cadherin expression and 17% (11/64) of tumors with normal E-cadherin expression. Expression of CD10 in tumor cells showed significant correlation with abnormal expression of E-cadherin (p=0.038) (Fig. 3).
According to results of the current study, CD10 expression was observed in 80% of non-neoplastic bladder urothelium. In addition, its degree was significantly higher in normal bladder mucosa, compared with TCCs. Conflicting results for CD10 expression in normal bladder epithelium have been reported. According to several previous reports, expression of CD10 was not observed in normal bladder urothelium [14-16]. In contrast, recent studies have reported that CD10 expression was observed in 50% of normal bladder urothelium and was successively lost from normal urothelium to TCCs [9,11]. The conflicting results may suggest that patients with normal bladder mucosa were exposed to different microenvironments and differed in their molecular profiles. CD10, a cell surface zinc-dependent metallopeptidase, is expressed in a variety of cell types [5-7]. CD10 is involved in regulation of vital physiological function through two major mechanisms [7]. First, CD10 cleaves peptides through its extracellular enzymatic activity. Second, CD10 is involved in an intracellular signaling pathway that interferes with other major signaling pathways controlled by elements present in the microenvironment. Deregulation of CD10 expression leads to accumulation or loss of peptides, disturbing regulation of cellular proliferation and differentiation, and deranges intracellular signaling pathways. A previous study reported a close correlation of CD10 expression with an increase in the rate of apoptosis in diffuse large B cell lymphoma [17]. In addition, CD10 suppresses growth of pancreas and lung cancer cell lines [18,19]. Based on the current results, as well as results of several recent studies, the possibility that CD10 expression may prevent normal bladder urothelium from carcinogenesis through degradation of molecules delivering anti-apoptotic signals and growth-promoting signals cannot be excluded.
Results of the current study demonstrated a significant correlation of CD10 expression with tumor grade and tumor invasion. Conflicting results for CD10 expression have also been reported with regard to the grade and invasion of TCC. A previous study reported an inverse correlation of CD10 expression with tumor stage and no association with tumor grade [8]. Another recent study demonstrated a higher degree of CD10 expression in primary tumor and tumor centers than in nodal metastatic foci and invasion front, respectively [9]. In addition, a favorable outcome in primary tumors with high CD10 expression has also been reported. In addition, CD10 expression was found to be a good prognostic marker in uterine cervical cancer and non-small cell lung cancer, suggesting that CD10 has an anti-tumor effect [20,21]. In addition, a recent study demonstrated that CD10-expressing fibroblasts induced by interleukin-1 produced by squamous cell carcinoma inhibited invasion of cancer cells through degradation of substance P [22]. The above mentioned results can be supported by the fact that CD10 is capable of degrading a number of bioactive peptides and cytokines necessary for growth of tumor cells. Therefore, the assumption that CD10 is expressed in high grade and invasive tumors in order to stop their progression cannot be excluded.
In contrast, several previous studies have reported correlation of CD10 expression with the grade and stage of TCC and shorter mean survival [10-12]. CD10 expression was also associated with liver metastasis and a decrease in the five-year disease-free survival rate in colon cancers [23]. In the current study, CD10 expression showed reciprocal correlation with membranous E-cadherin expression in tumors. Several studies have reported that the reduction of E-cadherin expression in TCC was closely associated with high grade and advanced stage as well as poor survival [24,25]. CD10 is a cell surface zinc-dependent metallopeptidase. MMPs can help cancer cells in invasion of adjacent extracelluar matrix via EMT through cleavage of E-cadherin and production of the activated splice variant Rac1b, stimulating elevation of the levels of cellular reactive oxygen species, which in turn leads to an increase in expression of Snail [3,4]. Overall, the possibility that CD10 expression may be correlated with progression of TCC is also considered.
Greater expression of CD10 was observed in non-neoplastic bladder urothelium, compared with TCCs, and its expression showed close correlation with the grade and invasion of TCCs. In addition, CD10 expression showed reciprocal correlation with expression of membranous E-cadherin in tumors. Overall, CD10 is again expressed at a certain stage during the neoplastic process of TCCs and could play some roles in their carcinogenesis.
References
1. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001; 17:463–516. PMID: 11687497.

2. Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002; 2:657–672. PMID: 12209155.

3. Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008; 25:593–600. PMID: 18286378.

4. Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997; 139:1861–1872. PMID: 9412478.

5. Malfroy B, Kuang WJ, Seeburg PH, Mason AJ, Schofield PR. Molecular cloning and amino acid sequence of human enkephalinase (neutral endopeptidase). FEBS Lett. 1988; 229:206–210. PMID: 3162217.

6. Erdos EG, Skidgel RA. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989; 3:145–151. PMID: 2521610.
7. Maguer-Satta V, Besancon R, Bachelard-Cascales E. Concise review: neutral endopeptidase (CD10): a multifaceted environment actor in stem cells, physiological mechanisms, and cancer. Stem Cells. 2011; 29:389–396. PMID: 21425402.

8. Bircan S, Candir O, Kapucuoglu N, Serel TA, Ciris M, Karahan N. CD10 expression in urothelial bladder carcinomas: a pilot study. Urol Int. 2006; 77:107–113. PMID: 16888412.

9. Seiler R, von Gunten M, Thalmann GN, Fleischmann A. High CD10 expression predicts favorable outcome in surgically treated lymph node-positive bladder cancer patients. Hum Pathol. 2012; 43:269–275. PMID: 21835428.

10. Bahadir B, Behzatoglu K, Bektas S, Bozkurt ER, Ozdamar SO. CD10 expression in urothelial carcinoma of the bladder. Diagn Pathol. 2009; 4:38. PMID: 19917108.

11. Murali R, Delprado W. CD10 immunohistochemical staining in urothelial neoplasms. Am J Clin Pathol. 2005; 124:371–379. PMID: 16191505.

12. Abdou AG. CD10 expression in tumour and stromal cells of bladder carcinoma: an association with bilharziasis. APMIS. 2007; 115:1206–1218. PMID: 18092952.

13. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998; 22:1435–1448. PMID: 9850170.
14. Koiso K, Akaza H, Ohtani M, Miyanaga N, Aoyagi K. A new tumor marker for bladder cancer. Int J Urol. 1994; 1:33–36. PMID: 7627835.

15. McIntosh GG, Lodge AJ, Watson P, Hall AG, Wood K, Anderson JJ, et al. NCL-CD10-270: a new monoclonal antibody recognizing CD10 in paraffin-embedded tissue. Am J Pathol. 1999; 154:77–82. PMID: 9916921.

16. Chu P, Arber DA. Paraffin-section detection of CD10 in 505 nonhematopoietic neoplasms. frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am J Clin Pathol. 2000; 113:374–382. PMID: 10705818.
17. Bai M, Agnantis NJ, Skyrlas A, Tsanou E, Kamina S, Galani V, et al. Increased expression of the bcl6 and CD10 proteins is associated with increased apoptosis and proliferation in diffuse large B-cell lymphomas. Mod Pathol. 2003; 16:471–480. PMID: 12748254.

18. Erhuma M, Kobel M, Mustafa T, Wulfanger J, Dralle H, Hoang-Vu C, et al. Expression of neutral endopeptidase (NEP/CD10) on pancreatic tumor cell lines, pancreatitis and pancreatic tumor tissues. Int J Cancer. 2007; 120:2393–2400. PMID: 17294442.

19. Ganju RK, Sunday M, Tsarwhas DG, Card A, Shipp MA. CD10/NEP in non-small cell lung carcinomas: relationship to cellular proliferation. J Clin Invest. 1994; 94:1784–1791. PMID: 7962523.

20. Terauchi M, Kajiyama H, Shibata K, Ino K, Mizutani S, Kikkawa F. Anti-progressive effect of neutral endopeptidase 24.11 (NEP/CD10) on cervical carcinoma in vitro and in vivo. Oncology. Oncology. 2005; 69:52–62. PMID: 16103735.
21. Tokuhara T, Adachi M, Hashida H, Ishida H, Taki T, Higashiyama M, et al. Neutral endopeptidase/CD10 and aminopeptidase N/CD13 gene expression as a prognostic factor in non-small cell lung cancer. Jpn J Thorac Cardiovasc Surg. 2001; 49:489–496. PMID: 11552274.
22. Xie L, Moroi Y, Tsuji G, Liu M, Hayashida S, Takahara M, et al. CD10-bearing fibroblast inhibits matrigel invasive potency of interleukin-1alpha-producing squamous cell carcinoma by diminishing substance P levels in the tumor microenvironment. Cancer Sci. 2010; 101:2570–2578. PMID: 20874839.
23. Fujita S, Taniguchi H, Yao T, Shimoda T, Ueno H, Hirai T, et al. Multi-institutional study of risk factors of liver metastasis from colorectal cancer: correlation with CD10 expression. Int J Colorectal Dis. 2010; 25:681–686. PMID: 20204382.

24. Baumgart E, Cohen MS, Silva Neto B, Jacobs MA, Wotkowicz C, Rieger-Christ KM, et al. Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res. 2007; 13:1685–1694. PMID: 17363521.

25. Jang TJ, Cha WH, Lee KS. Reciprocal correlation between the expression of cyclooxygenase-2 and E-cadherin in human bladder transitional cell carcinomas. Virchows Arch. 2010; 457:319–328. PMID: 20582552.

Fig. 1
(A) CD10 in non-neoplastic urothelium, (B) non-invasive low grade transitional cell carcinoma (TCC), (C) carcinoma in situ and (D) invasive high grade TCC (A-D, ×400), and positive rate of CD10 expression in non-neoplastic urothelium and bladder TCC (E, F). CD10 staining was predominantly cytoplasmic in tumor cells and non-neoplastic urothelium. CD10 expression was significantly higher in normal bladder mucosa, compared with TCC and also showed significant correlation with tumor grade and tumor invasion in TCC.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download