Introduction
Materials and Methods
1. Reagents
2. Cell culture
3. Transfection with siRNA for knockdown of MKP-1
4. Total RNA isolation and qRT-PCR analysis
5. Cell viability assay
6. Western blot analysis
7. Microarray data-set
8. Statistical analysis
Results
1. MKP-1 is highly expressed in human GBM tumors and GBM cell lines
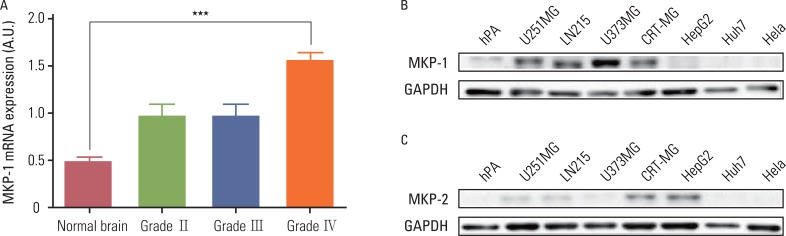 | Fig. 1MAP kinase phosphatase-1 (MKP-1) is highly expressed in human glioblastomas. (A) The expression of MKP-1 was evaluated using the microarray data of brain cancer patients provided by the Gene Expression Omnibus (GEO) database (GSE ID GSE2223). The results are presented as the means±SEM, and Tukey's post-hoc test was applied to significant group effects in ANOVA, ***p<0.001. (B) The expression of MKP-1 was measured by western blot analysis in human primary astrocytes, human glioblastomas (U251MG, LN215 and U373MG), human astroglioma (CRT-MG), human hepatomas (HepG2, Huh7), and human cervical adenocarcinoma (HeLa) cell lines. (C) The expression of MKP-2 was measured by western blot analysis in the same cell lines as in (B). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; hPA, human primary culturesd astrocytes. |
2. Downregulation of MKP-1 sensitizes GBM cells to cytotoxicity induced by chemotherapeutic agents
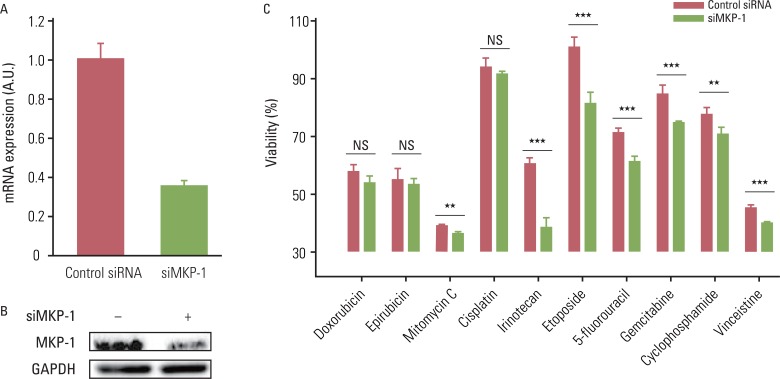 | Fig. 2The reduction of MAP kinase phosphatase-1 (MKP-1) expression increases sensitivity to anti-cancer drugs. (A) U251MG cells were transfected with control or MKP-1 specific siRNA (siMKP-1). After 48 hr of transfection, the transfection efficiency was determined by quantitative real-time polymerase chain reaction and normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. (B) MKP-1 protein expression was determined by western blot analysis. (C) Cells were treated with 10 different anti-cancer drugs 24 hr after siRNA transfection at the concentrations listed in Table 1. Cell viability was then evaluated by a WST-1 assay. The results are presented as the mean±SEM (n=4). Asterisks indicate a significant difference as determined by a Student's t-test, **p<0.01, ***p<0.001, and NS, not significant. Table 1The concentrations of anti-cancer drugs used in this study |
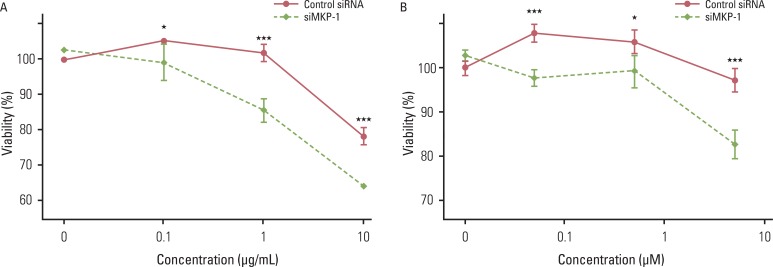 | Fig. 3The effect of MAP kinase phosphatase-1 (MKP-1) knockdown on irinotecan- and etoposide-induced cell death. Cells were transfected with control or MKP-1 specific siRNA (siMKP-1) for 48 hr, after which they were incubated with varying doses of irinotecan (A) or etoposide (B) for an additional 24 hr. Cell viability was evaluated by WST-1 assay. The results are presented as the mean±SEM (n=4). Asterisks indicate a significant difference as determined by a Student's t-test, *p < 0.05 and ***p < 0.001. |
3. The JNK pathway is involved in MKP-1-mediated tumor resistance to chemotherapeutic agents
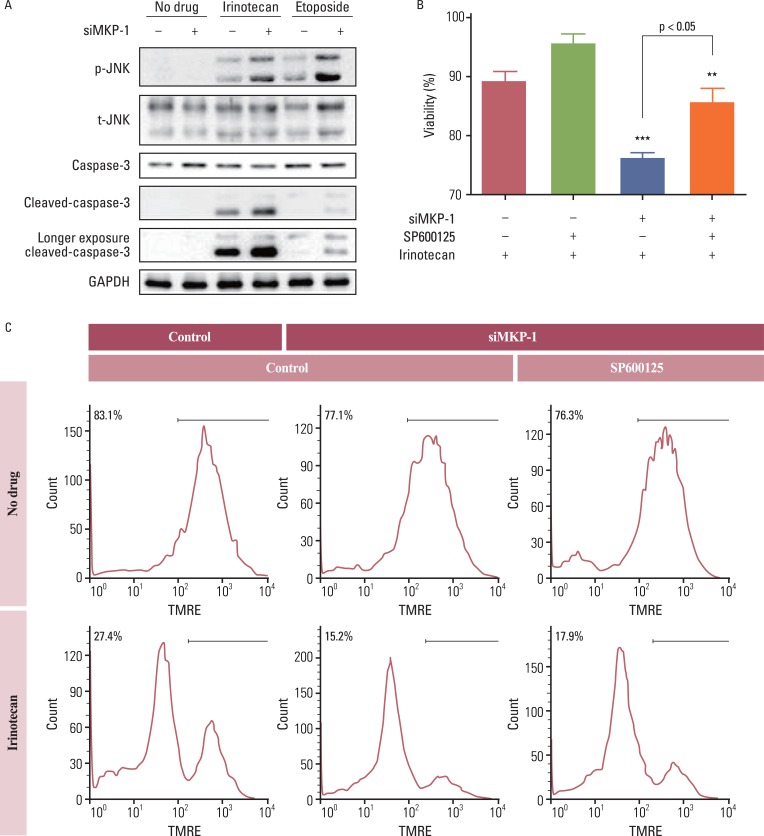 | Fig. 4The involvement of c-Jun N-terminal kinase (JNK) in irinotecan and etoposide-induced cell death. (A) Cells were transiently transfected with control or MAP kinase phosphatase-1 (MKP-1) specific siRNA (siMKP-1) for 48 hr, after which they were incubated in the presence or absence of irinotecan or etoposide (10 mg/L) for 15 min. Western blot analysis was used to determine the protein expression of phospho-JNK, total JNK, caspase-3, and cleaved caspase-3 in total cell lysates. (B) Cells were transiently transfected with control or siMKP-1 for 48 hr, after which they were incubated in the presence or absence of SP600125 (JNK inhibitor, 10 µmol/L) for 1 hr, and then treated in the presence or absence of irinotecan (10 mg/L) for an additional 24 hr. The cell viability was then evaluated by a WST-1 assay. The results are presented as the mean±SEM (n=4). Tukey's post-hoc test was applied to significant group effects in ANOVA, **p<0.01, ***p<0.001. (C) Cell viability was measured by flow cytometry after tetramethylrhodamine ethyl ester (TMRE; 100 nmol/L) staining. The labeled numbers indicate live cells. Images are representative of three independent experiments. p-JNK, phosphorylated JNK; t-JNK, total JNK; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download