This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The pattern of double primary cancers after treatment for gastric cancer is important for a patient's survival.
Materials and Methods
We analyzed the clinicopathologic data of 214 gastric cancer patients from October 1996 to November 2007 with regard to metachronous second primary cancers.
Results
Out of 5,778 patients with gastric cancer, metachronous second primary cancers occurred in 214 patients. The median age was 61.8 years, the number of male and female patients was 140 (65.4%), 74 (34.6%), respectively. The median time to the occurrence of second cancers after diagnosis of the first was 39.2 months (standard deviation, 31.2 months). The most common cancer was colorectal cancer, which occurred in 44 patients (20.6%), and lung cancer in 33 patients (15.4%), hepatocellular carcinoma in 26 patients (12.1%), ovarian cancer in 15 patients (7.0%), cervical cancer in 12 patients (7.0%), breast cancer in 11 patients (5.1%), and esophageal cancer in 11 patients (5.1%). The observed/expected (O/E) ratio showed a significant increase in colorectal (1.25), male biliary (1.60), ovarian (8.72), and cervical cancer (3.33) with primary gastric cancer. After five years from diagnosis of gastric cancer, secondary cancer occurred in 50 patients (23.4%), and breast cancer, prostate cancer, laryngeal cancer, lung cancer, and hepatocellular carcinoma were the most frequent.
Conclusion
The O/E ratio showed a significant increase in colorectal, male biliary, ovarian, and cervical cancer with primary gastric cancer, and second primary cancer as the main cause of death for these patients. A follow-up examination for metachronous double primary cancer is needed in order to improve the survival time in patients with gastric cancer.
Go to :

Keywords: Stomach neoplasm, Second primary neoplasms, Diagnosis
Introduction
Incidence of gastric cancer in Korea is very high, and, despite early detection, it continues to be a major health problem. Through achievement of remarkable advances in cancer treatment, the number of patients who survive cancer has shown a significant increase. One problem, however, is that because patients are living longer, they may be at increased risk of developing second primary cancer. The incidence of metachronous dual primary gastric cancer has increased with the concomitant increase in the prevalence of gastric cancer [
1]. Patients with metachronous cancer are defined as those with an interval of six months or more after diagnosis of gastric cancer [
2]. Some studies of double primary cancer in patients with gastric cancer have reported on prognosis or characteristics of metachronous cancer [
3-
5]. However, the general pattern of metachronous gastric cancer still needs to be clarified.
Due to the high incidence of gastric cancer in Korea, its surveillance in patients diagnosed with stomach cancer might be of clinical benefit. Although numerous reports on metachronous cancers have been issued, most have been simple case reports, as relatively few studies on the incidence and characteristics of metachronous cancers that develop during the postsurgical follow-up period have been conducted. Studies evaluating the characteristics of synchronous and metachronous cancer are limited, and the incidence of metachronous cancer varies to the extent that the clinical results differ in each study.
Further characterization of metachronous gastric cancer could provide valuable information for use in early diagnosis and treatment of these diseases. The purpose of this study was to investigate the prognosis and clinical features of metachronous gastric cancer.
Go to :

Materials and Methods
Between October 1996 and November 2007, 5,778 patients underwent surgical treatment or chemotherapy for gastric cancer at Dongsan Medical Center; however, these patients did not undergo endoscopic resection. Patients who had been diagnosed with new primary cancer developing from other origins were selected from the original group.
Among the 5,778 patients who underwent treatment for gastric cancer, metachronous cancers were diagnosed in 214 patients. The metachronous cancer patients described above could be defined as those with an interval of six months or more after diagnosis of gastric cancer [
2].
All patients with gastric cancer underwent routine preoperative upper gastrointestinal endoscopy. The histological diagnosis was established by gastrointestinal endoscopic biopsies and operative specimens. As part of the standard preoperative workup for gastric cancer, the staging workup included a complete physical examination, a complete blood count and biochemical profile, a gastrointestinal contrast study, and a chest and abdominal computed tomography scan. Second primary cancer was confirmed by histologic diagnosis, however, hepatocellular carcinoma, pancreatic cancer, and biliary cancer proved difficult to diagnose using this procedure in cases of poor performance. In such cases, second primary cancer was diagnosed through an imaging examination and tumor markers.
In addition, the age of patients at onset of metachronous cancer, or, the tumor, node and metastasis (TNM) stage, according to the American Joint Committee on Cancer (AJCC) 6th edition, and the presence of factors that could mediate effects on the survival rate were examined retrospectively. Evaluation of the interval from the first gastric surgery to development of second primary cancer, clinical characteristics, and survival rate after the second surgery was based on medical records.
1. Statistical analysis
The Kaplan-Meier method was used for generation of overall survival curves, and the log-rank test was used for comparison of between-group differences. Univariate and multivariate analyses with the Cox proportional hazard model were used for analysis of prognostic factors. The survival curve for patients with metachronous double primary cancer was calculated from the date of diagnosis of the second cancer. A p-value of <0.05 was considered to indicate statistical significance. The SPSS ver. 18.0 (SPSS Inc., Chicago, IL) was used.
Go to :

Results
Among 5,778 patients with gastric cancer, 209 patients (5.0%) were diagnosed with synchronous double primary cancer and 214 patients (3.7%) were diagnosed with metachronous double primary cancer. The group of patients consisted of 140 men and 74 women. Looking at the age distribution between the groups, the proportion was higher in older patients diagnosed with metachronous double primary cancer (p<0.001) (
Table 1). The most common group included patients aged 60 to 70, and the most common metachronous double primary cancer was colorectal cancer, followed by lung, liver (excluding cholangiocarcinoma), ovarian, and cervical cancer.
Table 1
Sex and age distribution of patients with metachronous double primary cancer in gastric cancer patients
|
Age (yr) |
With second cancera)
|
Without second cancer |
|
|
|
Male |
Female |
Male |
Female |
|
20-30 |
1 (5.3) |
0 (0) |
18 (94.7) |
39 (100) |
|
30-40 |
1 (0.6) |
6 (3.7) |
156 (99.4) |
158 (96.3) |
|
40-50 |
8 (1.6) |
15 (5.4) |
496 (98.4) |
264 (94.6) |
|
50-60 |
22 (2.5) |
17 (4.9) |
861 (97.5) |
329 (95.1) |
|
60-70 |
69 (5.1) |
24 (3.8) |
1,278 (94.9) |
601 (96.2) |
|
>70 |
39 (4.5) |
12 (2.2) |
832 (95.5) |
525 (97.8) |
|
Total |
140 (3.7) |
74 (3.7) |
3,641 (96.3) |
1,916 (96.3) |

Table 2 shows the mean age distribution of patients with metachronous double primary cancer. No difference was observed among the types of metachronous double primary cancer. The mean age was 61.8±10.62 years, and the oldest patient, who had lung cancer, was 68.5 years old, and the youngest patient, with breast cancer, was 51.7 years old.
Table 2
Cancer type and mean age distribution of metachronous double primary cancer in gastric cancer patients
|
Cancer type |
No. (%) |
Double primary cancer |
|
|
Age (mean±SD, yr) |
|
Colorectum |
44 (20.6) |
61.8±8.61 |
|
Lung |
33 (15.4) |
68.5±7.07 |
|
Liver |
26 (12.1) |
60.0±10.22 |
|
Ovary |
15 (7.0) |
54.6±12.45 |
|
Cervix |
12 (5.6) |
60.2±5.42 |
|
Breast |
11 (5.1) |
51.7±11.30 |
|
Esophagus |
11 (5.1) |
63.7±12.96 |
|
Biliary |
8 (3.7) |
67.6±7.73 |
|
Pancreas |
7 (3.3) |
58.6±8.94 |
|
Bladder |
6 (2.8) |
67.2±5.42 |
|
Skin |
5 (2.3) |
67.0±9.70 |
|
Larynx |
5 (2.3) |
67.2±10.18 |
|
Others |
31 (14.5) |
61.1±10.72 |
|
Total |
214 (100) |
61.8±10.62 |

In comparison of sex distribution of patients with metachronous double primary cancer, double primary cancer occurred more frequently in male patients, with the exception of breast, ovary, and cervical cancer (p<0.001) (
Table 3). The observed/expected (O/E) ratio was significantly higher in male colorectal (1.25), male biliary (1.60), ovarian (8.72), and cervical cancer (3.33) with primary gastric cancer, compared with double primary cancer.
Table 3
Type of metachronous double primary cancera) in gastric cancer patients according to sexb)
|
Cancer type |
Male (n=3,787) |
Female (n=1,991) |
Total (n=5,778)a)
|
|
Colorectum |
34 (0.90)b)
|
10 (0.05) |
44 (0.76) |
|
Lung |
28 (0.74) |
5 (0.25) |
33 (0.57) |
|
Liver |
23 (0.61) |
3 (0.15) |
26 (0.44) |
|
Ovary |
0 (0) |
15 (0.75)b)
|
15 (0.26) |
|
Cervix |
0 (0) |
12 (0.60)b)
|
12 (0.21) |
|
Breast |
0 (0) |
11 (0.55) |
11 (0.19) |
|
Esophagus |
11 (0.29) |
0 (0) |
11 (0.19) |
|
Biliary |
7 (0.18)b)
|
1 (0.05) |
8 (0.14) |
|
Others |
37 (0.98) |
17 (0.85) |
54 (0.93) |
|
Total |
140 (3.70) |
74 (3.72) |
214 (3.70) |

Table 4 shows the yearly diagnostic period for metachronous double primary cancer, and does not indicate any notable difference in incidence from year to year. The most common period of occurrence was 1-2 years (n=60, 40.8%). Subjective symptoms were observed in three patients after 10 years. These patients were diagnosed with hepatocellular carcinoma, lung cancer, and cervical cancer. The mean diagnostic period was 39.2±31.2 months. No significant differences were observed among sex and cancer groups. Bladder cancer and esophageal cancer were diagnosed earlier, compared to other cancers (
Table 5).
Table 4
Diagnostic period of metachronous double primary cancer in patients with gastric cancer
|
Diagnostic period (yr) |
No. |
|
<1 |
37 |
|
1-2 |
60 |
|
2-3 |
28 |
|
3-4 |
24 |
|
4-5 |
15 |
|
5-6 |
13 |
|
6-7 |
9 |
|
7-8 |
10 |
|
8-9 |
10 |
|
9-10 |
5 |
|
>10 |
3 |
|
Total |
214 |

Table 5
Mean diagnostic period of metachronous double primary cancer in gastric cancer patients
|
Cancer type |
Male |
Female |
Total |
|
Colorectum |
34.9±24.3 |
49.7±32.8 |
38.2±26.8 |
|
Lung |
43.2±35.9 |
45.1±43.1 |
43.4±36.3 |
|
Liver |
41.2±31.3 |
64.5±47.7 |
44.8±33.1 |
|
Ovary |
– |
34.0±28.1 |
34.0±28.1 |
|
Breast |
– |
59.0±35.2 |
59.0±35.2 |
|
Cervix |
– |
37.8±32.7 |
37.8±32.7 |
|
Esophagus |
24.5±27.7 |
– |
24.5±27.7 |
|
Biliary |
50.8±31.1 |
36.0 |
48.9±29.2 |
|
Pancreas |
39.7±21.6 |
27.9±15.2 |
34.7±18.7 |
|
Bladder |
24.0±13.4 |
17.3±12.3 |
22.9±12.3 |
|
Skin |
36.3±35.2 |
9.5 |
36.9±34.6 |
|
Larynx |
43.7±38.7 |
– |
43.7±38.7 |
|
Total |
38.5±30.8 |
40.7±32.1 |
39.2±31.2 |

Table 6 shows development of metachronous double primary cancer over a period of fiveyears, after diagnosis of gastric cancer. Development of breast cancer (54.5%), prostate cancer (50.0%), and laryngeal cancer (40.0%) occurred much more frequently after five years from diagnosis of gastric cancer.
Table 6
Diagnosis of metachronous double primary cancer after five years from diagnosis of gastric cancer
|
Cancer type |
No. (%) |
Total |
|
Colorectum |
10 (22.7) |
44 |
|
Lung |
10 (30.3) |
33 |
|
Liver |
7 (26.9) |
26 |
|
Breast |
6 (54.5) |
11 |
|
Cervix |
2 (16.7) |
12 |
|
Biliary |
2 (25.0) |
8 |
|
Larynx |
2 (40.0) |
5 |
|
Ovary |
2 (13.3) |
15 |
|
Prostate |
2 (50.0) |
4 |
|
Others |
7 (12.5) |
56 |
|
Total |
50 (23.4) |
214 |

The mean follow-up period was 35.5 months for metachronous cancers. The median survival period for metachronous double primary cancer after diagnosis of second primary cancer was 19.8±3.33 months (
Table 7). The four year survival rate for patients with double primary gastric cancer was 4%, however, the rate without second primary cancer was 61.1% (
Fig. 1). The median survival period for patients with breast, laryngeal, cervical, and colorectal cancers was longer than that of other cancers, however, no survival difference was observed according to gastric cancer stage (
Table 8).
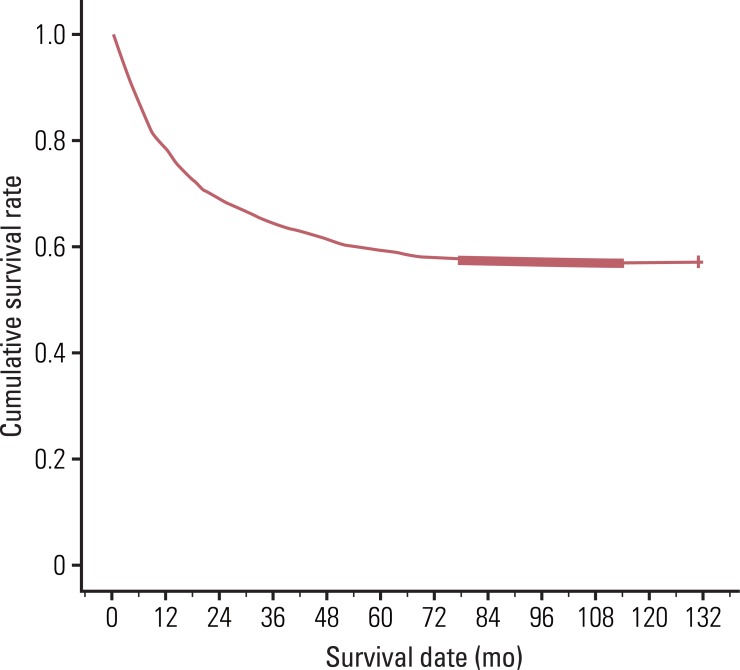 | Fig. 1Four-year survival rate for gastric cancer patients without double primary cancer. 
|
Table 7
Median survival after diagnosis of metachronous double primary cancer in patients with gastric cancer
|
Cancer type |
No. |
Survival (median±SD, mo)a)
|
|
Colorectum |
44 |
25.5±3.85 |
|
Lung |
33 |
13.3±5.73 |
|
HCC |
26 |
9.6±12.54 |
|
Ovary |
15 |
17.4±5.13 |
|
Cervix |
12 |
30.6±21.22 |
|
Breast |
11 |
53.1±13.73 |
|
Esophagus |
11 |
19.3±22.36 |
|
Biliary |
8 |
8.8±6.21 |
|
Pancreas |
7 |
3.0±3.37 |
|
Bladder |
6 |
15.5±39.14 |
|
Skin |
5 |
25.0±12.60 |
|
Larynx |
5 |
32.6±13.77 |
|
Others |
31 |
9.2±3.90 |
|
Total |
214 |
19.8±3.33 |

Table 8
Median survival of metachronous cancer according to gastric cancer stage
|
Cancer stage |
No. |
After gastric cancer diagnosis (mo) |
After double primary cancera) (mo) |
|
I |
114 |
62.03 |
23.90 |
|
II |
54 |
111.83 |
33.70 |
|
III |
32 |
44.90 |
25.50 |
|
IV |
2 |
124.93 |
14.87 |
|
I-II |
168 |
67.40 |
25.30 |
|
III-IV |
34 |
44.9 |
17.37 |

Go to :

Discussion
The first study of synchronous and metachronous cancer in patients with gastric cancer was reported by Yoshino et al. [
6]. Results of the study, conducted in Japan, showed an incidence of synchronous and metachronous cancer in patients with gastric cancer of 2.0%. Other studies have since reported incidence rates of 1.1-4.7% [
3-
10]. In this study, the incidence was 3.7%, which appears to be overestimated, as it included patients who had not been diagnosed with pathologic confirmation due to their physical condition and/or had difficulty in receiving a pathologic diagnosis.
In this study, the most common double primary cancer was colorectal cancer, followed by lung, liver (excluding cholangiocarcinoma), ovary, and cervical cancer. Comparing this result with the general cancer incidence in Korea, the incidence of colorectal cancer is higher than expected. That is, although colorectal cancer is the third most common cancer in the general population, it is the most common double primary cancer. This result has been observed not only in Korea, but also in Japan, China, and Australia. In the general population, colorectal cancer is the fifth most common cancer in China and the third most common cancer in Australia; however, it is the most common double primary cancer in both countries [
4,
11,
12]. Many papers have evaluated genetic and environmental relationships between gastric and colorectal cancer [
13,
14]. Therefore, performance of regular colonoscopies on patients who have been diagnosed with gastric cancer is important, particularly for those who are over sixty. The median age for secondary gastric cancer was 63.0 years; the number of males was 137, the number of females was 59. Hepatocellular carcinoma (30 in 2,481 cases, 1.21%), esophageal cancer (24 in 518 cases, 4.63%), and lung cancer (20 in 2,814 cases, 0.71%) were reported as common types of primary cancers.
A comparison of clinicopathological characteristics among patients with other primary cancers and patients without them was conducted by Kato et al. [
15] and Lee et al. [
9]. Statistical differences in age, sex, and gastric cancer staging were observed in these previous studies. That is, the mean age, sex ratio, and proportion of early gastric cancer was higher in patients with other primary cancers than in those without. This study reported similar results, except with regard to gastric cancer staging; no significant difference was observed in gastric cancer staging, compared to the statistics.
The incidences of most cancers are higher in males than in females and tend to increase with age. Patients with metachronous cancer of ovary or breast were younger than those with other cancers, which means that female patients with metachronous double primary cancer tend to be younger than male patients. In this study, the O/E ratio was found to be significantly higher in colorectal, biliary, ovarian, and cervical cancer with primary gastric cancer. In order to enhance detection and survival rate, conduct of a large scale prospective study is necessary for investigation of the efficacy of colonoscopies, abdomen and pelvic computed tomographys, and PAP smears.
Previous studies have reported that the majority of double primary malignancies develop within a period of three years, and that surveillance gastroscopy is commonly recommended for a period of three years after surgery for treatment of colorectal cancer [
16]. However, there was no exact surveillance colonoscopy recommendation period or surveillance tool for detection of colon cancer after gastric cancer surgery. A higher percentage of metachronous cancers was confirmed for both colorectal and lung cancer. Ikeda et al. [
4] reported that metachronous lung cancer showed a high incidence of advanced-stage disease and was a major cause of death in patients with metachronous double primary cancers. Therefore, close attention should be paid and regular checkups should be performed on patients who have undergone surgery for treatment of gastric cancer.
Patients who have not been diagnosed with secondary cancers within five years after gastric cancer surgery are usually regarded as having been cured. In this study, metachronous double primary cancer was found to occur after 10 years; therefore, performance of regular checkups for a longer period of time on patients who have undergone surgery for treatment of gastric cancer is important.
Survival rates of patients with a metachronous or synchronous cancer were analyzed by Maehara et al. [
5], Furukawa et al. [
17], and Ikeda et al. [
4]. According to the study reported by Ikeda et al. [
4], the 10-year survival rate was 69.3% for gastric cancer patients without a double primary cancer, 40.1% for patients with synchronous double primary cancer, and 75.2% for patients with metachronous double primary cancer. In this study, the median survival time for patients with metachronous double primary cancer after diagnosis of second primary cancer was 19.8 months, and 55.2 months after diagnosis of gastric cancer. Survival rates for patients withpancreatic cancer and hepato-biliary cancer were very low, indicating a strong relationship of survival rate with the type of double primary cancer. Other prognostic factors should be evaluated in order to demonstrate the difference in median survival rate of patients with metachronous double primary cancer.
Go to :

Conclusion
Patients with gastric cancer develop double primary cancers either synchronously or metachronously, and, in the present study, second primary cancer was the main cause of death for these patients. The O/E ratio showed a significant increase in colorectal, male biliary, ovarian, and cervical cancer with primary gastric cancer, and second primary cancer was the main cause of death for these patients. A follow-up examination for metachronous double primary cancer is needed in order to improve the survival time in patients with gastric cancer.
Go to :

Acknowledgments
This study was supported by a grant from the Korea Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A091326).
Go to :

Notes
Go to :

References
1. Watanabe M, Kochi M, Fujii M, Kaiga T, Mihara Y, Funada T, et al. Dual primary gastric and colorectal cancer: Is the prognosis better for synchronous or metachronous? Am J Clin Oncol. 2011; 6. 08. [Epub].
http://dx.doi.org/10.1097/COC.0b013e318218585a
.
2. Moertel CG, Bargen JA, Dockerty MB. Multiple carcinomas of the large intestine: a review of the literature and a study of 261 cases. Gastroenterology. 1958; 34:85–98. PMID:
13501357.
3. Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, et al. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008; 98:106–110. PMID:
18452218.

4. Ikeda Y, Saku M, Kawanaka H, Nonaka M, Yoshida K. Features of second primary cancer in patients with gastric cancer. Oncology. 2003; 65:113–117. PMID:
12931016.

5. Maehara Y, Tomisaki S, Emi Y, Sakaguchi Y, Kusumoto T, Ichiyoshi Y, et al. Clinicopathological features of patients who died with second primary cancer after curative resection for gastric cancer. Anticancer Res. 1995; 15:1049–1053. PMID:
7645924.
6. Yoshino K, Asanuma F, Hanatani Y, Otani Y, Kumai K, Ishibiki K. Multiple primary cancers in the stomach and another organ: frequency and the effects on prognosis. Jpn J Clin Oncol. 1985; 15(Suppl 1):183–190. PMID:
4009981.
7. Lundegardh G, Hansson LE, Nyren O, Adami HO, Krusemo UB. The risk of gastrointestinal and other primary malignant diseases following gastric cancer. Acta Oncol. 1991; 30:1–6. PMID:
2009177.

8. Lynge E, Jensen OM, Carstensen B. Second cancer following cancer of the digestive system in Denmark, 1943-80. Natl Cancer Inst Monogr. 1985; 68:277–308. PMID:
4088303.
9. Lee JH, Bae JS, Ryu KW, Lee JS, Park SR, Kim CG, et al. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol. 2006; 12:2588–2592. PMID:
16688807.

10. Hiyama T, Hanai A, Fujimoto I. Second primary cancer after diagnosis of stomach cancer in Osaka, Japan. Jpn J Cancer Res. 1991; 82:762–770. PMID:
1908843.

11. Yang L, Parkin DM, Ferlay J, Li L, Chen Y. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev. 2005; 14:243–250. PMID:
15668501.
12. Heard A, Roder D, Luke C. Multiple primary cancers of separate organ sites: implications for research and cancer control (Australia). Cancer Causes Control. 2005; 16:475–481. PMID:
15986102.

13. Deakin M, Elder J, Hendrickse C, Peckham D, Baldwin D, Pantin C, et al. Glutathione S-transferase GSTT1 genotypes and susceptibility to cancer: studies of interactions with GSTM1 in lung, oral, gastric and colorectal cancers. Carcinogenesis. 1996; 17:881–884. PMID:
8625505.
14. Knekt P, Jarvinen R, Dich J, Hakulinen T. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int J Cancer. 1999; 80:852–856. PMID:
10074917.
15. Kato I, Kito T, Nakazato H, Tominaga S. Second malignancy in stomach cancer patients and its possible risk factors. Jpn J Clin Oncol. 1986; 16:373–381. PMID:
3795533.
16. Yun HR, Yi LJ, Cho YK, Park JH, Cho YB, Yun SH, et al. Double primary malignancy in colorectal cancer patients: MSI is the useful marker for predicting double primary tumors. Int J Colorectal Dis. 2009; 24:369–375. PMID:
18797888.
17. Furukawa H, Hiratsuka M, Iwanaga T, Imaoka S, Kabuto T, Ishikawa O, et al. Treatments for second malignancies after gastrectomy for stomach cancer. Hepatogastroenterology. 1996; 43:194–198. PMID:
8682461.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download