Abstract
Clinical observation of skin metastasis in ovarian cancer cases is relatively uncommon. And distant metastatic skin lesions including the extremities are much rarer still as most metastatic skin lesions are located in the skin in the abdominal wall adjacent to where the primary ovarian tumors exist. We report the case of a 60-year-old woman who presented skin lesions on both lower extremities as a consequence of the metastasis of serous papillary adenocarcinoma of the ovary. She presented with erythematous and painful cutaneous nodules on both upper legs and in the inguinal area 42 months after initial diagnosis of ovarian cancer. Skin biopsy revealed metastasis of adenocarcinoma in the dermis. She was treated with surgical excision and systemic chemotherapy. Literature review has suggested that a combined modality approach including surgical excision and chemotherapy may be useful in the management of skin metastases due to ovarian cancer.
Epithelial ovarian carcinoma is the leading cause of death from gynecologic malignancy [1]. The disease usually remains confined to the peritoneal cavity at presentation. And although the intraperitoneal route of dissemination is considered the most common, ovarian cancer may also metastasize through the lymphatic channels and the hematogenous route [2]. The most common sites of distant metastasis are the pleura, liver, lung and lymph nodes. However, skin metastasis occurs only in 3.5% of patients with ovarian carcinoma [2]. It has been reported that only 12% of cases of cutaneous metastasis due to ovarian carcinoma occur on the limbs, as most metastatic skin lesions occur in skin adjacent to the primary ovarian cancer to include the abdominal wall [3]. In Korea, there have been only two case reports on skin metastasis of primary ovarian carcinoma [4,5] and none reported the presentation of metastatic skin lesions on the limbs. Herein, we report a case of International Federation of Gynecological Oncologists (FIGO) stage IIIC serous papillary ovarian cancer which recurred as a cutaneous nodule on the upper leg at 42 months after primary surgery.
The patient was a 60-year-old women, gravida 5, para 3, who was referred to our hospital with suspected ovarian cancer in June 2006. The patient's preoperative cancer antigen 125 level was 916.0 U/mL. On July 3, 2006 she underwent exploratory laparotomy. Approximately 4×4 cm sized bilateral ovarian tumors were firmly attached to the uterine wall and serosa of the recto-sigmoid colon. Approximately 7×6 cm sized omental cake was observed and miliary seedings were visible on the bowel, mesentery, diaphragm, abdominal peritoneum and posterior cul-de-sac. Her surgical stage was determined to be FIGO stage IIIc. A total abdominal hysterectomy including both salpingo-oophorectomy and total omentectomy were performed, concluding as suboptimal debulking with residual masses more than 1 cm on the right diaphragm, posterior cul-de-sac, and on the surface of the sigmoid colon. Before the end of the surgery we placed a drain through the abdominal wall. Histologic examination revealed serous papillary adenocarcinoma with grade 3 differentiation. Postoperatively, she received six cycles of adjuvant chemotherapy consisting of carboplatin (AUC 5) and docetaxel (75 mg/m2) every three weeks. All evidence of disease disappeared after six cycles of chemotherapy and she was considered a complete response. Her first recurrence was diagnosed 12 months after her last chemotherapy treatment. Most of the recurrent lesions were found in the supraclavicular, right axilla, mediastinum and right hilar lymph nodes. She was treated with five cycles of carboplatin (AUC 5) and paclitaxel (175 mg/m2). Progressive lymphadenopathy including new lesions in the inguinal and pelvic lymph nodes were observed during second-line chemotherapy. Until November 6, 2009, in order to evaluate the tumor response, the chemotherapy regimen was changed several times to include belotecan (1.5 mg/m2) with carboplatin (AUC 5), docetaxel (75 mg/m2) with carboplatin (AUC 5), and etoposide (100 mg/m2) with ifosfamide (1.0 g/m2). In addition, she was treated with palliative radiotherapy for refractory lesions. A total dose of 30 Gy was administered by 10 fractions on lymph nodes including the right axilla, subclavicular, left and right inguinal, and pelvic. After combined treatment of chemotherapy and radiotherapy with partial response, due to chemotherapy induced hematologic toxicity, she spent one month without treatment.
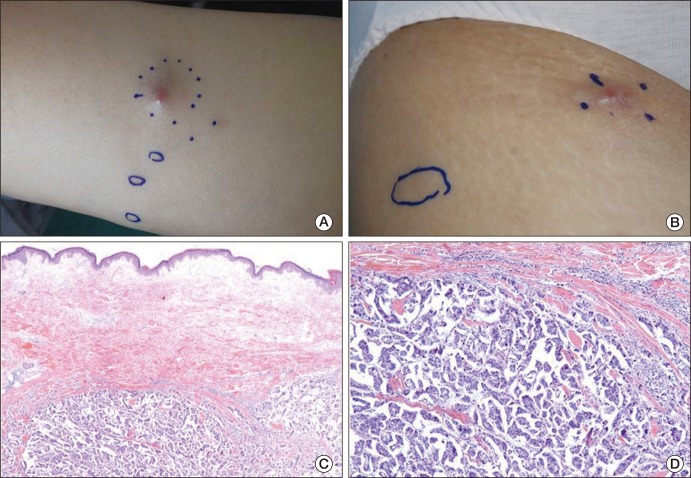
In December 2009, approximately 42 months after the initial diagnosis, she presented with nodular skin lesions located at the right thigh and right inguinal areas (Fig. 1A and B). The skin lesions were multiple, erythematous, up to 2 cm in size, and she complained of accompanying pain and itching. These symptoms were not able to be controlled by supportive care with medication. Biopsy of the lesions revealed metastatic serous adenocarcinoma (Fig. 1C and D). Based on the evidence from a positron emission tomography-computerized tomography scan, significant disease progression had occurred in both cervical, axilla, left internal mammary, left back area, subcarinal, upper abdomen, retroperitoneum, mesentery, pelvic cavity, left buttock, both inguinal and proximal thigh, right psoas and right iliacus muscle. Palliative chemotherapy consisted of gemcitabine (750 mg/m2/wk) and carboplatin (AUC 4), administered starting January 3, 2010.
During chemotherapy, in February 2010, symptoms of pain and itching on the skin lesions on both the thigh and inguinal area were aggravated. On March 4, 2010, skin metastaectomy was performed under general anesthesia for symptomatic relief and for the aim of removing chemotherapy resistant tumors. Overlying skin was incised in a semicircular fashion and all masses were dissected and removed. The subcutaneous layer was sutured with Vicryl 2-0 and skin was sutured with Dermalone 2-0. As expected, the pathology results revealed metastatic serous adenocarcinoma. After metastaectomy, the symptoms related to the skin lesions markedly improved. On April 21, 2010, abrupt left sided weakness developed, a complication of leptomeningeal seeding and subsequent meningitis. Starting May 5, 2010 left sided seizure like movements appeared and electroencephalography results revealed severe diffuse cerebral dysfunction. On May 30, 2010, she died from the disease progression.
Effective chemotherapy for the treatment of ovarian carcinoma has prolonged patient survival, allowing metastases in rare distant sites to implant, grow, and become a clinically evident disease [6]. Our literature review suggested that the incidence of skin metastasis is rare and ranges from 1.9% to 5.1% [7-9], and furthermore, metastatic skin lesions on extremities are much rarer still. In the case series we evaluated which reported the largest number (n=9) of skin metastasis due to ovarian cancer, most of the patients had skin lesions on the abdominal wall (n=6) and chest wall (n=3). None of the patients had skin lesions of the lower extremities [7]. The abdominal lesions were associated with scars from surgery including abdominal wall incision or from placing drainage. Of the several mechanisms which explain the occurrence of skin metastasis, the particular findings in these cases may have resulted from the direct spread of tumor cells from the underlying growth, accidental implantation associated with surgery, and contiguous spread of tumor cells through lymphatic or hematogenous routes [8,10]. As shown in our case, isolated skin lesions on the lower extremities without the existence of abdominal skin lesions may have resulted by tumor embolus through the lymphatic channel or by hematogenous routes which often manifest with a virtually limitless and unpredictable pattern of cutaneous localization [11].
In almost all carcinomas, the cutaneous metastases tend to appear late in the course of the disease and are usually associated with poor prognosis [8,9,11]. In a previous report, performance status, the presence of other sites of disease, and the interval of time between the diagnosis of ovarian cancer and the occurrence of distant metastases were the only significant factors associated with survival [12]. In our case, the interval of time between the diagnosis of ovarian cancer and the observation of skin metastasis was 42 months, similar to the 44 month average (range, 3 to 105 months) reported in a previous series [12].
No standard treatment exists for extra-abdominal metastases resulting from epithelial ovarian carcinoma. Besides chemotherapy, which is the main palliative treatment, symptomatic management of extra-abdominal metastases should be considered. And in the case of skin metastasis, treatment options depend on the patients' subjective symptoms and the number and localization of the lesions. For local cutaneous disease, surgical resection may need to be considered. However, considering that the treatment of recurrent ovarian cancer is palliative, surgical excision should be considered in selected patients after evaluating the patient's general condition and life expectancy. Skin lesions on the extremities may be safer to remove by surgery than abdominal skin lesions, which are typically associated with intra-abdominal lesions. For extensive cutaneous metastasis, it has been reported that electrocoagulation or external radiotherapy may be effective for local control with less pain, hemorrhage and infection, but these approaches also have limitation in adequately treating systemic disease [13,14].
In conclusion, we reported the first case of metastatic skin lesions on the lower extremities due to ovarian cancer in a Korean woman. Skin metastases on the lower limbs of ovarian cancer patients have been rarely reported globally, and when it is reported, the prognosis is poor. There is no current standard treatment. Although it is unclear whether or not multimodal treatment including surgery and chemotherapy can increase the duration of survival, these treatments may be recommended for the relief of symptoms in select patients with skin metastasis resulting from recurrent ovarian carcinoma.
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249. PMID: 19474385.

2. Dauplat J, Hacker NF, Nieberg RK, Berek JS, Rose TP, Sagae S. Distant metastases in epithelial ovarian carcinoma. Cancer. 1987; 60:1561–1566. PMID: 3621129.

3. Brownstein MH, Helwig EB. Metastatic tumors of the skin. Cancer. 1972; 29:1298–1307. PMID: 4336632.

4. Kim YJ, Kim SK, Kim HD, Cho MK, Park YL, Lee JS, et al. A case of skin metastasis of ovarian cancer (mucinous cystadenocarcinoma). Korean J Dermatol. 2010; 48:342–345.
5. Kwon SY, Hur YM, Park HK, Nam KH, Jin SY, Lee MC, et al. A case of recurrent ovarian carcinoma metastasizing to umbilical skin mass. Korean J Obstet Gynecol. 1992; 35:1528–1532.
6. Cheng B, Lu W, Xiaoyun W, YaXia C, Xie X. Extra-abdominal metastases from epithelial ovarian carcinoma: an analysis of 20 cases. Int J Gynecol Cancer. 2009; 19:611–614. PMID: 19509558.
7. Cormio G, Capotorto M, Di Vagno G, Cazzolla A, Carriero C, Selvaggi L. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003; 90:682–685. PMID: 13678747.

8. Spencer PS, Helm TN. Skin metastases in cancer patients. Cutis. 1987; 39:119–121. PMID: 3829718.
10. Braverman IM. Cutaneous signs of systemic disease. Med Times. 1977; 105:82–95. PMID: 875677.
11. Krumerman MS, Garret R. Carcinomas metastatic to skin. N Y State J Med. 1977; 77:1900–1903. PMID: 270017.
12. Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003; 13:125–129. PMID: 12657111.

13. Patsner B, Mann WJ, Chumas J, Loesch M. Herpetiform cutaneous metastases following negative second look laparatomy for ovarian adenocarcinoma. Arch Gynecol Obstet. 1988; 244:63–67. PMID: 3240006.
14. Tapley ND. Radiation therapy with the electron beam. Semin Oncol. 1981; 8:49–58. PMID: 6787709.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download