Abstract
A compelling body of non-randomized evidence has established stereotactic ablative lung radiotherapy (SABR) as a standard of care for medically inoperable patients with peripheral early-stage non-small cell lung cancer (NSCLC). This convenient outpatient therapy, which is typically delivered in 3-8 fractions, is also well tolerated by elderly and frail patients, makes efficient use of resources and is feasible using standard commercial equipment. The introduction of lung SABR into large populations has led to an increased utilization of radiotherapy, a reduction in the proportion of untreated patients and an increase in overall survival. In selected patients, the same ablative technology can now achieve durable local control of NSCLC metastases in a variety of common locations including the adrenal glands, bone, brain, and liver. At the same time as this, advances in prognostic molecular markers and targeted systemic therapies mean that there is now a subgroup of patients with stage IV NSCLC and a median survival of around 2 years. This creates opportunities for new trials that incorporate SABR and patient-specific systemic strategies. This selective mini-review focuses on the emerging role of SABR in patients with early-stage and oligometastatic NSCLC.
Stereotactic ablative radiotherapy (SABR), which is also referred to as stereotactic body radiotherapy (SBRT), is a form of high-precision radiotherapy (RT). It is typically delivered in one to 8 intermittent fractions, taking up to 2.5 weeks to complete and commonly using fraction sizes of 7.5-20 Gy or more. This compares with 20 or more fractions of 3 Gy or less taking up to 7 weeks to complete that is typical of conventional RT (Fig. 1) [1,2]. Modern lung SABR is characterized by patient-specific planning techniques that account for individual tumor motion whilst delivering heterogeneous dose distributions to the tumor and sparing normal tissues; accurate and reproducible imageguided patient setup prior to and during each fraction; and 'beam-on' radiation delivery times that can be 10 minutes or less with some techniques [3,4]. SABR can be delivered using standard commercial equipment, and is readily incorporated into departmental workflows.
Local control rates of 90% or more are now possible with SABR for peripheral stage I non-small cell lung cancer (NSCLC) measuring up to 5 cm in maximum diameter [5,6]. The key to achieving this is believed to be a sufficiently high radiation dose delivered in only a few fractions, combined with accurate tumour targeting [7,8]. These results contrast impressively with those of conventional RT using doses of at least 50-60 Gy in fraction sizes of 3 Gy or less, for which local failure rates of 40-50% and more can be expected [9,10]. The combination of improved local control and an acceptable toxicity profile mean that in recent years, SABR has become the preferred treatment for patients with medically inoperable stage I NSCLC in countries like the Netherlands and Japan [11,12]. There are several important sources of evidence to support this strategy. In addition to the institutional and phase II studies already cited and a recent meta-analysis [13], there is now encouraging population-based data [11]. In this latter study of patients aged 75-years or older, the introduction of SABR led to a 16% absolute increase in utilization of RT, a decline in the proportion of untreated elderly patients, and an improvement in overall survival. This was statistically significant only for the subgroup treated using RT, in which there was an estimated median increase in overall survival of 6 months, from 20 to 26 months, between cohorts of patients treated in 1999-2001 and 2005-2007. The survival benefits observed in this population-based cancer registry are plausible as SABR has been shown to be well tolerated in elderly patients [14], and in those with severe chronic obstructive airways disease [15,16]. Both the baseline and post-SABR cohorts are from the period after which fluorodeoxyglucose (FDG) positron emission tomography (PET) scanning became widely available, increasing the likelihood that SABR may be responsible for the improved outcomes. Although there have been suggestions that higher quality evidence is needed to confirm the improved therapeutic ratio for SABR over conventional RT before it enters routine use [17] a randomized trial comparing these two treatment approaches seems unlikely (Table 1) [18-22]. Indeed a convincing argument could now be made against such a trial on the grounds of a lack of uncertainty and clinical (community) equipoise [23]. Where SABR is not available or accepted, it should be noted that hypo-fractionated conventionally delivered RT is another option that might also deliver improvements in local control. For example, 48-60 Gy in fractions of 4 Gy has been associated with actuarial local control rates of 70% at 5 years [18].
In the protracted debate over supportive evidence, it is appropriate to reflect on the seriousness of a diagnosis of lung cancer for today's patients, who require access to the best currently available treatment. Analysis of the Surveillance, Epidemiology and End Results (SEER) registry using data from elderly patients (>65 years of age) diagnosed with stage I or II NSCLC between 1992-2002 who were not treated by surgery, found that if they also had no RT, their median survival was 7 months [24]. This could be increased by 6 months, to 13 months, when they received RT, however only 59% of patients did so. Overall, 71% of patients died of lung cancer progression. A recent Dutch analysis showed that 38% patients of elderly patients presenting with stage I NSCLC in the period between 1999-2001 received no local therapy [11]. Many factors may influence the uptake of a treatment. For example, protracted courses of conventional RT may be perceived as ineffective, inconvenient and poorly tolerated by frail patients and their clinicians. SABR addresses all of these concerns. Furthermore, guidelines and training [3,25] combined with advances in technology and quality assurance procedures may improve knowledge and increase confidence, facilitating the adoption of new techniques. A recent survey of SABR in the United States highlights other important drivers including research (for academic radiation oncologists), and competitive reasons (for those in private practice) [26]. However, despite such levers, introducing technology into some healthcare systems has been a major challenge. It is easy to overlook the fact that the process needs to be actively managed with advance planning, clear priorities and carefully considered implementation strategies [27].
One concern expressed by some has been the wide range of dose-fractionation schemes reported in the lung SABR literature. However, there is now appreciable clinical data from a variety of sources to indicate that local control is compromised when a biological effective dose (BED) of less than 100 Gy10 is delivered, calculated using an alpha/beta ratio of 10 Gy [28-30]. Data from our own center reports consistently high rates of initial tumor control across a range of 'riskadapted' dose-fractionation schemes, all with a BED≥105 Gy10 [6]. Caution is therefore required in advocating doses of less than 100 Gy10 for peripheral lung SABR of targets up to 5 cm in diameter, especially when considering that such schedules are associated with low rates of significant toxicity [31,32]. Indeed, high-grade toxicity and mortality is uncommon following SABR for peripheral lung tumours, and rib fractures and radiation pneumonitis have generally occurred in less than 10% of patients (although the former may depend on such factors as the proximity to the chest wall, dose-fractionation scheme and planning/delivery technique) [31,32]. Damage to the brachial plexus has been reported in patients treated for apical tumors, particularly when plexus doses exceeded a BED of 100 Gy3 (i.e., calculated using an alpha/beta ratio of 3 Gy) [33]. It remains to be seen if the incidence of these complications will be reduced by the use of lower fraction sizes for selected tumors located closer to critical structures. Such a risk-adapted strategy was adopted when our lung SABR program started in 2003, taking in to account factors such as tumor size/treatment volume, the location of the target volume and the extent of contact with the chest wall [6]. In some centers patients with tumors >5 cm and also those that are centrally located are now being treated with risk-adapted SABR [34]. Follow up durations are currently limited, and it should be noted that the safety profile of SABR for central and to some extent larger tumors is likely to be different and so neither the clinical toxicity data, nor many of the treatment schedules for peripheral lung SABR should be directly extrapolated to these scenarios. One example concerns central airway toxicity, which has been reported after SABR for lung tumors extending into the mediastinum [35,36].
The role of non-surgical treatment options in patients who are potentially fit to undergo surgery has increasingly been the subject of discussion [37,38]. Recent reports of SABR have included small numbers of patients who had declined to undergo surgery, and two prospective single-arm trials of SABR in patients who are fit to undergo surgery have completed accrual (JCOG 0403/NCT00238875 and RTOG 0618/NCT00551369). A Markov model analysis of outcomes after either SABR or lobectomy for stage I NSCLC for a 5-year time frame indicated that SABR might offer comparable overall survival and quality-adjusted life expectancy as compared with surgical resection [39]. This is consistent with a matched propensity analysis from Washington University of 57 high-risk surgical patients and 57 patients undergoing SABR for stage I NSCLC, which found comparable local control and disease specific survival between the two groups at 3 years [40]. Importantly, both the 5- and 3-year time points used in these studies are considerably longer than the median time to loco-regional recurrence in patients who have had surgery for early-stage NSCLC (see below) [41]. Current changes in demography mean that more than half of new patients with lung cancer are older than 70 years, and the absolute number of elderly patient is projected to increase faster than the increase in the overall population [42]. Extrapolating from a small sub-group analysis, SABR might be a particularly attractive treatment option for elderly patients as the benefits of adjuvant chemotherapy for patients with occult nodal metastases detected in stage I NSCLC resection were not apparent [43]. Furthermore, even in a study where the median patient age was 64 years, only 66% of patients who were candidates for adjuvant chemotherapy post-surgery actually started chemotherapy [44].
Two issues that remain to be resolved in the coming years are techniques for improving methods for the diagnosis of peripheral lung tumours and for detecting local recurrence after SABR. Obtaining a pre-treatment diagnosis in patients presenting with a peripheral lung nodule suspicious for a lung cancer can be challenging, particular as patients who are medically inoperable due to co-morbid disease, may be at higher risk for complications following a trans-thoracic needle biopsy. The likelihood of lung malignancy in this setting can be calculated using a combination of clinical, radiological and PET findings [45]. Applying this approach to SABR appears justified in a country such as the Netherlands where a diagnosis of benign disease is typically made in less than 5% of patients undergoing surgery [46-48]. However this policy may not be well suited to populations with a higher likelihood of benign lung disease. Indeed, in some populations the use of only a single computed tomography and PET scan without other supportive data can lead to nearly one third of resected PET-positive nodules turning out to be granulomas [49]. Improved diagnostic algorithms are clearly necessary in such populations.
The pattern of disease failure after lung SABR is now well described. Distant metastases predominate. In one study actuarial distant failure-free survival rates of 85% and 77% were reported 1 and 2 years post-SABR [6]. The rate of regional failure in patients staged with FDG-PET is generally around 10% [31]. For patients who are fit enough for potential salvage options, the early identification of local recurrence is important. However post-SABR pulmonary changes may be complex and can sometimes be hard to distinguish from tumor progression [50]. This necessitates careful patient follow-up and access to specialist multi-disciplinary teams who are familiar with interpreting post treatment imaging. At the present time, one observation that may help to discriminate local recurrence from post-SABR change is that the former may be characterized by the rapid enlargement of a mass within a relatively short period of time after lung SABR. This was the case in recent reports describing experience with salvage surgery [51,52] and is consistent with the timing of local-regional failure after surgery. For example, a retrospective study of 975 patients undergoing surgical resection for pathological stage I/II NSCLC between 1995-2005 found that the median time from surgery to loco-regional failure was 13.9 months [41]. Data such as these may help to guide optimal follow up strategies, however they need to be complemented by improvements in imaging and image analysis to discriminate between recurrent tumor and post-SABR changes. The feasibility of surgical salvage as a treatment option for recurrences post-SABR requires further study, particularly as it may increase the preference for SABR in some patients who are fit to undergo primary surgery.
Looking ahead, there are now a number of randomized clinical trials comparing SABR and surgery but the results will not be available for many years to come. The use of lung SABR for patients with medically operable disease generates discussions about the relative risks of the two interventions. In a recent population based analysis from the Netherlands, lung SABR for medically inoperable patients was associated with a 1% 30-day mortality when it was largely delivered in only 2 regional centers [11]. This is similar in magnitude to the post-operative 30-day mortality reported in the ACOSOG Z0030 study of pulmonary resection for early-stage lung cancer in medically operable patients [53]. While results from individual centers can be expected to vary, a population-based study using SEER data has linked an increased risk of operative mortality to several factors, including increased age and certain comorbidities [54]. This emphasizes the importance of risk stratification and both expanding and individualizing the therapeutic options for all patients with lung cancer. The leading role played by the surgical community in studying the impact of practice organization and operating protocols on patient outcome is well recognized [55,56] and as lung SABR diffuses into routine clinical practice throughout academic and community centers it will also become important to learn from this experience and consider similar issues in radiation oncology.
As survival in patients with stage IV NSCLC has historically been considered to be poor, low-dose palliative RT has been the standard of care in patients with symptomatic disease [57]. Justification for such an approach was found for example in the outcome of a large phase III trial comparing 4 different third-generation platinum-based chemotherapy schemes [58]. This Eastern Cooperative Oncology Group study reported a response rate for all 1,155 eligible patients of 19%, with a median survival of 7.9 months, a 1-year survival rate of 33% (95% confidence interval, 30 to 36%), and a 2-year survival rate of 11% (95% confidence interval, 8 to 12%). Such results indicated that only a minority of patients would present with recurrent disease requiring re-irradiation, and if needed repeat low-dose palliative RT was the prevailing policy. A change in thinking regarding the management of metastatic NSCLC lung cancer came about with several publications, including those dating from the 1990's when long-term survival was noted selected patients who underwent resection (or rarely RT) for limited volume metastatic disease, in combination with radical management of the primary tumor [59,60].
Other studies have also suggested an advantage for ablative management strategies in selected patients with advanced NSCLC. In patients who present with newly diagnosed NSCLC and synchronous brain metastases, a retrospective study in 167 patients from our own institution has suggested that in a subgroup of selected patients aged 65 years or younger, who were also eligible to undergo surgery or radiosurgery for their brain metastases, radical treatment for their thoracic disease may be justified [61]. This strategy is indirectly supported by a systematic review of randomized controlled trials of palliative thoracic RT which concluded that improvements in survival were more likely with higher-dose RT to the primary tumour (BED≥35 Gy10) [62]. Further support is also provided by the observation that the predominant pattern of failure in patients with advanced NSCLC after first-line systemic therapy may be at the sites of known disease [63]. In keeping with the view that cancer is a spectrum, it has been hypothesized that the most relevant issue may not be whether or not there are distant metastases, but instead what the ratio is between local and systemic disease [64]. In this case, it is possible that the impact of chemotherapy may be fairly similar for tumours that are either primarily localized or systemic, whereas the benefit of adding chest RT could increase as the proportion of tumour cells that are localized increases.
Many institutional reports now show surprisingly long survival outcomes in 'definitively managed' patients with metastatic NSCLC [64], and recent guidelines, including those from the British Thoracic Society, support the need for clinical trials of radical treatment for patients with M1 disease [65]. At present, several non-invasive treatment techniques, including SABR can be used to ablate metastases. Reports evaluating SABR in the treatment of metastases to the liver [66], adrenal gland (Fig. 2) [67], lung [68], brain [69], and bone (Fig. 3) [70], have reported high rates of local control, sometimes in excess of 90% when ablative doses of RT have been administered. Lower radiation doses have been associated with lower rates of tumor control. Together with recent developments in SABR, this has lead to a resurgence of interest in the treatment of oligometastatic disease, a state characterized by a limited number of clinically apparent metastases with the possibility that these might genuinely represent the only distant sites of disease [71,72]. Such treatment paradigms support a move away from dichotomous definitions of treatments as curative or palliative, encouraging a more useful spectral interpretation of interventions and their potential for life-prolongation [32,63], as well as debates about how best to integrate an increasing range of possible therapies.
Work is needed to identify who might benefit most from intensified management strategies. One strategy, the identification of molecular signatures that can augment clinical features, is a priority. In recent randomized trials, treatment of NSCLC with activated mutations of the epidermal growth factor receptor (EGFR) has shown response rates in excess of 60%, and median survival exceeding 24 months [73,74]. The identification of this and other molecular subtypes of stage IV disease [75], where prolonged disease control can be attained using targeted agents is now rapidly transforming the systemic treatment of stage IV NSCLC. Studies evaluating the additional role of ablative therapies in such patients with limited volume oligometastatic disease are a logical step (Table 2), including in East Asian populations who have a higher frequency of EGFR (and other) mutations [76]. Ultimately, one aim of such treatment paradigms would be to achieve long-term survival, although a less-controversial first step may be to prolong life while also preserving quality and function [64]. A number of issues need to be resolved before larger-scale trials can be initiated. Phase I-II data is required to establish the toxicity of both sequential and concurrent administration of molecularly targeted agents and high dose RT, particularly as unexpected toxicities have been encountered [77]. In addition, innovative and careful trial design is likely to be necessary to increase the chance of successfully answering important clinical questions [78]. Simultaneously, it is also necessary to continue to refine treatment techniques and quality assurance strategies that support the safe, efficient and effective delivery of SABR to single and multiple targets [79-81].
In summary, at the present time the available data justify the use of ablative therapies such as SABR for treating selected patients with early-stage and advanced NSCLC. While the history of surgical resection for colorectal cancer liver metastases suggests that efforts to radically change existing treatment paradigms in NSCLC are likely to encounter scepticism and require time [82], for certain indications, in particular lung SABR for early stage peripheral NSCLC in medically inoperable patients, the field may already have moved beyond the need for randomized controlled trials [83]. While there is a legitimate debate to be had over the appropriate methodologies and standards of evidence with which to assess clinical interventions and advanced RT technologies [22], this should not overshadow the needs of today's patients, nor should it deter immediate and pragmatic steps to responsibly integrate promising treatments and useful technologies into clinical practice.
References
1. Loo BW, Chang JY, Dawson LA, Kavanagh BD, Koong AC, Senan S, et al. Stereotactic ablative radiotherapy: whats in a name? Pract Radiat Oncol. 2011; 1:38–39.

2. Palma D, Senan S. Stereotactic radiation therapy: changing treatment paradigms for stage I nonsmall cell lung cancer. Curr Opin Oncol. 2011; 23:133–139. PMID: 21107257.

3. Hurkmans CW, Cuijpers JP, Lagerwaard FJ, Widder J, van der Heide UA, Schuring D, et al. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiat Oncol. 2009; 4:1. PMID: 19138400.

4. Verbakel WF, Senan S, Cuijpers JP, Slotman BJ, Lagerwaard FJ. Rapid delivery of stereotactic radiotherapy for peripheral lung tumors using volumetric intensity-modulated arcs. Radiother Oncol. 2009; 93:122–124. PMID: 19552979.

5. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010; 303:1070–1076. PMID: 20233825.

6. Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008; 70:685–692. PMID: 18164849.

7. van Sörnsen de Koste JR, Lagerwaard FJ, Nijssen-Visser MR, Graveland WJ, Senan S. Tumor location cannot predict the mobility of lung tumors: a 3D analysis of data generated from multiple CT scans. Int J Radiat Oncol Biol Phys. 2003; 56:348–354. PMID: 12738308.

8. Underberg RW, Lagerwaard FJ, Cuijpers JP, Slotman BJ, van Sörnsen de Koste JR, Senan S. Four-dimensional CT scans for treatment planning in stereotactic radiotherapy for stage I lung cancer. Int J Radiat Oncol Biol Phys. 2004; 60:1283–1290. PMID: 15519801.

9. Lagerwaard FJ, Senan S, van Meerbeeck JP, Graveland WJ. Rotterdam Oncological Thoracic Study Group. Has 3-D conformal radiotherapy (3D CRT) improved the local tumour control for stage I non-small cell lung cancer. Radiother Oncol. 2002; 63:151–157. PMID: 12063004.

10. Cheung PC, Yeung LT, Basrur V, Ung YC, Balogh J, Danjoux CE. Accelerated hypofractionation for early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002; 54:1014–1023. PMID: 12419427.

11. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010; 28:5153–5159. PMID: 21041709.

12. Nagata Y, Hiraoka M, Mizowaki T, Narita Y, Matsuo Y, Norihisa Y, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D Conformal External Beam Radiotherapy Group. Int J Radiat Oncol Biol Phys. 2009; 75:343–347. PMID: 19735861.

13. Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbonions for non-small cell lung cancer: a meta-analysis. Radiother Oncol. 2010; 95:32–40. PMID: 19733410.

14. Haasbeek CJ, Lagerwaard FJ, Antonisse ME, Slotman BJ, Senan S. Stage I nonsmall cell lung cancer in patients aged > or =75 years: outcomes after stereotactic radiotherapy. Cancer. 2010; 116:406–414. PMID: 19950125.
15. Louie AV, Rodrigues G, Hannouf M, Lagerwaard FJ, Palma D, Zaric GS, et al. Withholding stereotactic radiotherapy in elderly patients with stage I non-small cell lung cancer and co-existing COPD is not justified: outcomes of a Markov Model analysis. Radiother Oncol. 2011; 99:161–165. PMID: 21620503.

16. Palma D, Lagerwaard FJ, Rodrigues G, Haasbeek CJ, Senan S. Curative treatment of stage I non-small cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. 2011; 6. 01. [Epub]. Doi: 10.1016/j.ijrobp.2011.03.005
.
17. Ball D. Extracranial stereotactic body radiotherapy for stage I non-small cell lung cancer: still investigational or standard of care? J Thorac Oncol. 2008; 3:1209–1210. PMID: 18978553.

18. Soliman H, Cheung P, Yeung L, Poon I, Balogh J, Barbera L, et al. Accelerated hypofractionated radiotherapy for early-stage non-small-cell lung cancer: long-term results. Int J Radiat Oncol Biol Phys. 2011; 79:459–465. PMID: 20385455.

19. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010; 28:1117–1123. PMID: 20124165.

20. Mintz A, Perry J, Spithoff K, Chambers A, Laperriere N. Management of single brain metastasis: a practice guideline. Curr Oncol. 2007; 14:131–143. PMID: 17710205.

21. Grossman J, Mackenzie FJ. The randomized controlled trial: gold standard, or merely standard? Perspect Biol Med. 2005; 48:516–534. PMID: 16227664.

22. Bentzen SM. Radiation oncology health technology assessment: the best is the enemy of the good. Nat Clin Pract Oncol. 2008; 5:563. PMID: 18818689.
23. Weijer C, Shapiro SH, Cranley Glass K. For and against: clinical equipoise and not the uncertainty principle is the moral underpinning of the randomised controlled trial. BMJ. 2000; 321:756–758. PMID: 10999914.
24. Wisnivesky JP, Halm E, Bonomi M, Powell C, Bagiella E. Effectiveness of radiation therapy for elderly patients with unresected stage I and II non-small cell lung cancer. Am J Respir Crit Care Med. 2010; 181:264–269. PMID: 19892859.

25. Hurkmans CW, van Lieshout M, Schuring D, van Heumen MJ, Cuijpers JP, Lagerwaard FJ, et al. Quality assurance of 4D-CT scan techniques in multicenter phase III trial of surgery versus stereotactic radiotherapy (radiosurgery or surgery for operable early stage (Stage 1A) non-small-cell lung cancer [ROSEL] study). Int J Radiat Oncol Biol Phys. 2011; 80:918–927. PMID: 20950961.

26. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011; 3. 15. [Epub]. Doi: 10.1002/cncr.26067
.

27. Dahele M, Senan S. Radiation oncology: overview and recent advances. J R Coll Physicians Edinb. 2010; 40:136–143. PMID: 21125059.

28. Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007; 2(7 Suppl 3):S94–S100. PMID: 17603311.

29. Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008; 70:847–852. PMID: 18262098.

30. Olsen JR, Robinson CG, El Naqa I, Creach KM, Drzymala RE, Bloch C, et al. Dose-response for stereotactic body radiotherapy in early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011; 4. 06. [Epub]. Doi: 10.1016/j.ijrobp.2011.01.038
.

31. Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010; 94:1–11. PMID: 20074823.

32. Milano MT, Constine LS, Okunieff P. Normal tissue toxicity after small field hypofractionated stereotactic body radiation. Radiat Oncol. 2008; 3:36. PMID: 18976463.

33. Forquer JA, Fakiris AJ, Timmerman RD, Lo SS, Perkins SM, McGarry RC, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol. 2009; 93:408–413. PMID: 19454366.

34. Ong CL, Palma D, Verbakel WF, Slotman BJ, Senan S. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): planning considerations and early toxicity. Radiother Oncol. 2010; 97:431–436. PMID: 20971523.

35. Song SY, Choi W, Shin SS, Lee SW, Ahn SD, Kim JH, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009; 66:89–93. PMID: 19168260.

36. Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006; 24:4833–4839. PMID: 17050868.

37. Haasbeek CJ, Senan S, Smit EF, Paul MA, Slotman BJ, Lagerwaard FJ. Critical review of nonsurgical treatment options for stage I non-small cell lung cancer. Oncologist. 2008; 13:309–319. PMID: 18378542.

38. Timmerman RD. Surgery versus stereotactic body radiation therapy for early-stage lung cancer: who's down for the count? J Clin Oncol. 2010; 28:907–909. PMID: 20065172.

39. Louie AV, Rodrigues G, Hannouf M, Zaric GS, Palma DA, Cao JQ, et al. Stereotactic body radiotherapy versus surgery for medically operable stage I non-small-cell lung cancer: a Markov Model-based decision analysis. Int J Radiat Oncol Biol Phys. 2010; 10. 05. [Epub]. Doi: 10.1016/j.ijrobp.2010.06.040
.

40. Crabtree TD, Denlinger CE, Meyers BF, El Naqa I, Zoole J, Krupnick AS, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010; 140:377–386. PMID: 20400121.

41. Boyd JA, Hubbs JL, Kim DW, Hollis D, Marks LB, Kelsey CR. Timing of local and distant failure in resected lung cancer: implications for reported rates of local failure. J Thorac Oncol. 2010; 5:211–214. PMID: 19901853.

42. Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008; 100:888–897. PMID: 18544740.

43. Pepe C, Hasan B, Winton TL, Seymour L, Graham B, Livingston RB, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol. 2007; 25:1553–1561. PMID: 17442999.

44. Felip E, Rosell R, Maestre JA, Rodríguez-Paniagua JM, Morán T, Astudillo J, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol. 2010; 28:3138–3145. PMID: 20516435.

45. Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest. 2005; 128:2490–2496. PMID: 16236914.
46. van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002; 359:1388–1393. PMID: 11978336.

47. Herder GJ, Kramer H, Hoekstra OS, Smit EF, Pruim J, van Tinteren H, et al. Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: a Dutch cooperative randomized study. J Clin Oncol. 2006; 24:1800–1806. PMID: 16567772.

48. Belgers EH, Siebenga J, Bosch AM, van Haren EH, Bollen EC. Complete video-assisted thoracoscopic surgery lobectomy and its learning curve: a single center study introducing the technique in The Netherlands. Interact Cardiovasc Thorac Surg. 2010; 10:176–180. PMID: 19850598.

49. May BJ, Levsky JM, Godelman A, Jain VR, Little BP, Mahadevia PS, et al. Should CT play a greater role in preventing the resection of granulomas in the era of PET? AJR Am J Roentgenol. 2011; 196:795–800. PMID: 21427327.

50. Dahele M, Palma D, Lagerwaard F, Slotman B, Senan S. Radiological changes following stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol. 2011; 5. 26. [Epub]. Doi: 10.1097/JTO.0b013e318219aac5
.
51. Neri S, Takahashi Y, Terashi T, Hamakawa H, Tomii K, Katakami N, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol. 2010; 5:2003–2007. PMID: 21102262.

52. Chen F, Matsuo Y, Yoshizawa A, Sato T, Sakai H, Bando T, et al. Salvage lung resection for non-small cell lung cancer after stereotactic body radiotherapy in initially operable patients. J Thorac Oncol. 2010; 5:1999–2002. PMID: 21102261.

53. Allen MS, Darling GE, Pechet TT, Mitchell JD, Herndon JE 2nd, Landreneau RJ, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006; 81:1013–1019. PMID: 16488712.

54. Kates M, Perez X, Gribetz J, Swanson SJ, McGinn T, Wisnivesky JP. Validation of a model to predict perioperative mortality from lung cancer resection in the elderly. Am J Respir Crit Care Med. 2009; 179:390–395. PMID: 19029001.

55. O'Connor GT, Plume SK, Olmstead EM, Morton JR, Maloney CT, Nugent WC, et al. The Northern New England Cardiovascular Disease Study Group. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. JAMA. 1996; 275:841–846. PMID: 8596221.
56. Finley CJ, Bendzsak A, Tomlinson G, Keshavjee S, Urbach DR, Darling GE. The effect of regionalization on outcome in pulmonary lobectomy: a Canadian national study. J Thorac Cardiovasc Surg. 2010; 140:757–763. PMID: 20850656.
57. Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997; 15:2996–3018. PMID: 9256144.
58. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98. PMID: 11784875.

59. Burt M, Wronski M, Arbit E, Galicich JH. Resection of brain metastases from nonsmall-cell lung carcinoma. Results of therapy. Memorial Sloan-Kettering Cancer Center Thoracic Surgical Staff. J Thorac Cardiovasc Surg. 1992; 103:399–410. PMID: 1312184.
60. Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg. 1996; 62:1614–1616. PMID: 8957360.

61. Lind JS, Lagerwaard FJ, Smit EF, Postmus PE, Slotman BJ, Senan S. Time for reappraisal of extracranial treatment options? Synchronous brain metastases from nonsmall cell lung cancer. Cancer. 2011; 117:597–605. PMID: 20872880.
62. Fairchild A, Harris K, Barnes E, Wong R, Lutz S, Bezjak A, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008; 26:4001–4011. PMID: 18711191.

63. Rusthoven KE, Hammerman SF, Kavanagh BD, Birtwhistle MJ, Stares M, Camidge DR. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol. 2009; 48:578–583. PMID: 19373699.

64. Marks LB, Saynak M, Christodouleas JP. Stage III vs. stage IV lung cancer: "Crossing a Great Divide". Lung Cancer. 2010; 67:1–3. PMID: 19962209.

65. Lim E, Baldwin D, Beckles M, Duffy J, Entwisle J, Faivre-Finn C, et al. Guidelines on the radical management of patients with lung cancer. Thorax. 2010; 65(Suppl 3):iii1–iii27. PMID: 20940263.

66. Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009; 27:1572–1578. PMID: 19255321.

67. Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys. 2011; 2. 05. [Epub]. Doi: 10.1016/j.ijrobp.2010.11.060
.

68. Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009; 27:1579–1584. PMID: 19255320.

69. Wegner RE, Olson AC, Kondziolka D, Niranjan A, Lundsford LD, Flickinger JC. Stereotactic radiosurgery for patients with brain metastases from small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011; 2. 22. [Epub]. Doi: 10.1016/j.ijrobp.2011.01.001
.

70. Lovelock DM, Zhang Z, Jackson A, Keam J, Bekelman J, Bilsky M, et al. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys. 2010; 77:1282–1287. PMID: 20350795.

72. Mehta N, Mauer AM, Hellman S, Haraf DJ, Cohen EE, Vokes EE, et al. Analysis of further disease progression in metastatic non-small cell lung cancer: implications for locoregional treatment. Int J Oncol. 2004; 25:1677–1683. PMID: 15547705.

73. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009; 361:958–967. PMID: 19692684.

74. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957. PMID: 19692680.

75. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363:1693–1703. PMID: 20979469.
76. Sun Y, Ren Y, Fang Z, Li C, Fang R, Gao B, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010; 28:4616–4620. PMID: 20855837.

77. Soria JC, Deutsch E. Hemorrhage caused by antiangiogenic therapy within previously irradiated areas: expected consequence of tumor shrinkage or a warning for antiangiogenic agents combined to radiotherapy? Ann Oncol. 2011; 22:1247–1249. PMID: 21382869.

78. Senan S, Smit EF. Design of clinical trials of radiation combined with antiangiogenic therapy. Oncologist. 2007; 12:465–477. PMID: 17470689.

79. Klein EE, Hanley J, Bayouth J, Yin FF, Simon W, Dresser S, et al. Task Group 142 report: quality assurance of medical accelerators. Med Phys. 2009; 36:4197–4212. PMID: 19810494.
80. Kuijper IT, Dahele M, Senan S, Verbakel WF. Volumetric modulated arc therapy versus conventional intensity modulated radiation therapy for stereotactic spine radiotherapy: a planning study and early clinical data. Radiother Oncol. 2010; 94:224–228. PMID: 20122745.

81. Dahele M, Zindler JD, Sanchez E, Verbakel WF, Kuijer JP, Slotman BJ, et al. Imaging for stereotactic spine radiotherapy: clinical considerations. Int J Radiat Oncol Biol Phys. (in press).

82. Woodington GF, Waugh JM. Results of resection of metastatic tumors of the liver. Am J Surg. 1963; 105:24–29. PMID: 14010473.

83. Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006; 55(Suppl 3):iii1–iii8. PMID: 16835351.

Fig. 1
This peripheral stage I lung tumor (left) was treated using 3 fractions of 18 Gy. The panel on the right side shows the colorwash representing high-dose regions within the planning target volume (red contour).
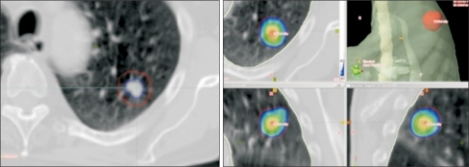
Fig. 2
This patient had initially undergone resection of a T2N0 primary non-small cell lung cancer and single fraction stereotactic radiosurgery for 2 synchronous brain metastases. Eleven mo later, after radiosurgery to 2 more brain metastases, they developed a solitary metastasis in the right adrenal gland measuring 5.3 cm in diameter (left, arrow), which was treated with stereotactic ablative lung radiotherapy to a dose of 60 Gy in 8 fractions. The 60 Gy dose (colored area, middle panel) tightly covers the treatment volume. A computed tomography scan 4 mo later shows a reduction in diameter to 3.3 cm (right, arrow).
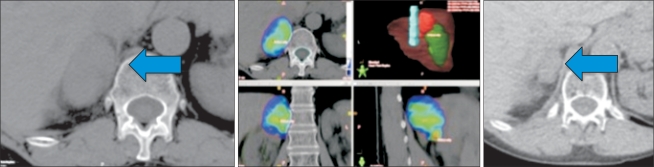
Fig. 3
This patient had previously undergone a single fraction of palliative radiotherapy for a painful vertebral metastasis, and subsequently developed progressive local pain. A magnetic resonance imaging scan (left) revealed progressive vertebral destruction and thecal sac/spinal cord compression. Whilst respecting the tolerance of the spinal cord, stereotactic re-irradiation delivering a minimum dose of 8 Gy per fraction to most of the tumor volume (colorwash) was possible. The patient received 2 fractions, and the maximum point dose in the tumor was 13 Gy per fraction.
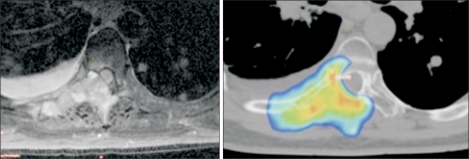
Table 1
Reasons why a randomized clinical trial of conventional radiotherapy vs. SABR for medically inoperable peripheral stage I NSCLC may be unlikely to succeed
Table 2
Examples of potential patient groups and clinical scenarios for whom routine clinical treatment or studies with SABR may be appropriate




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download