Introduction
Materials and Methods
1. Generation of αDC1s
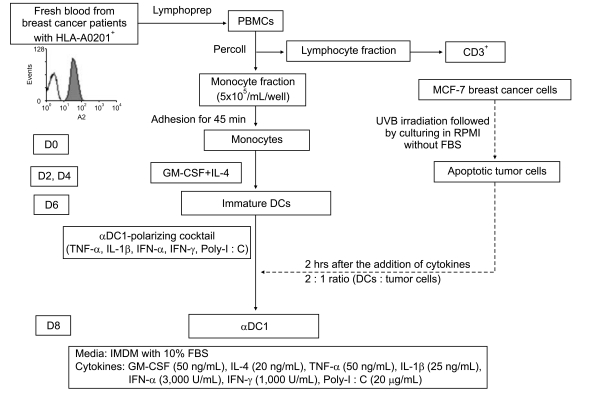 | Fig. 1Schema of the generation of dendritic cells (DCs) in patients with breast cancer in this study. PBMCs, peripheral blood mononuclear cells; D, day; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; poly-I : C, polyinosinic : polycytidylic acid; αDC1, alpha-type 1 polarized DCs; IMDM, Iscove's Modified Dulbecco's medium; FBS, fetal bovine serum; UVB, ultraviolet B. |
2. Preparation of the UVB-irradiated tumor cells as an antigen source
3. Preparation of breast cancer cells
4. Phenotypic expression of αDC1s and tumor-antigen uptake
5. Cytokine analyses by enzyme-linked immunosorbent assay (ELISA)
6. Allogeneic T cell stimulatory capacities
7. In vitro priming of CD4+CD45RA+ naïve T cells with DCs
8. Induction of breast-cancer-specific CTLs
Results
1. Characteristics of mature and polarized DCs in patients with breast cancer
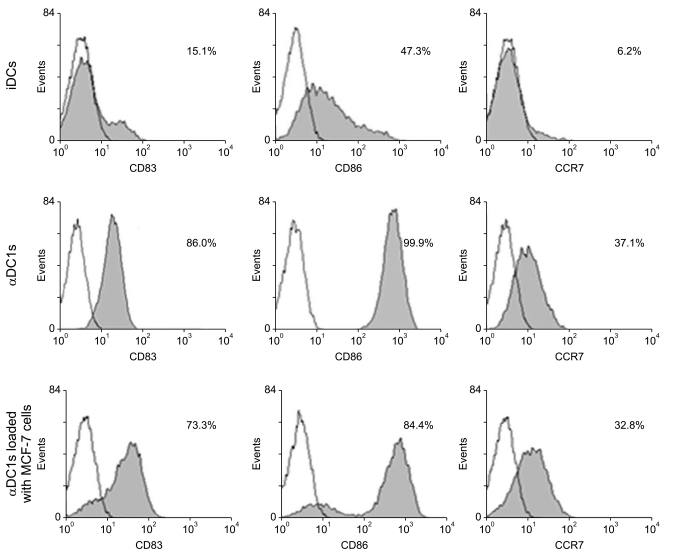 | Fig. 2Phenotypic expression of dendritic cells (DCs). The expressions of several molecules (CD83, CD86 and CCR7) related to DC maturation were significantly higher in the alpha-type 1 polarized DCs (αDC1s) than in the immature DCs (iDCs). However, there were no differences in phenotypic expression between the αDC1s and the αDC1s loaded with apoptotic MCF-7 breast cancer cells. Data are from one representative experiment of four independent experiments. |
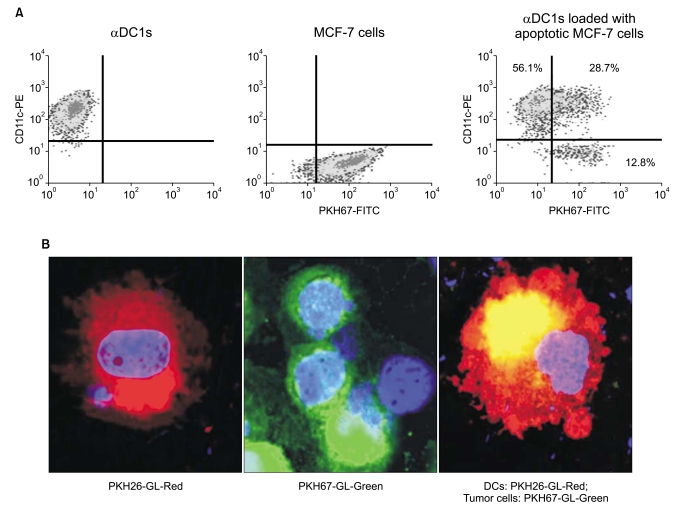 | Fig. 3Efficacy of tumor antigen uptake by dendritic cells (DCs). DCs were loaded with PKH67-labeled UVB-irradiated MCF-7 breast cancer cells (a ratio of 2 : 1) 2 hr after the addition of maturation-inducing cytokines on day 6. Mature DCs were harvested on day 8 and identified by CD11c expression. (A) The tumor antigen uptake of the alpha-type 1 polarized DCs (αDC1s) was measured by the percent of double-positive cells using flow cytometry. (B) After labeling MCF-7 cells with PKH67-GL-Green and DCs with PKH26-GL-Red, cell morphology was observed by using a confocal microscope at an excitation wavelength of 350 nm for DAPI. Apoptotic MCF-7 cells (yellow) were confirmed in the αDC1s. |
2. High level of IL-12p70 production by αDC1s in breast cancer
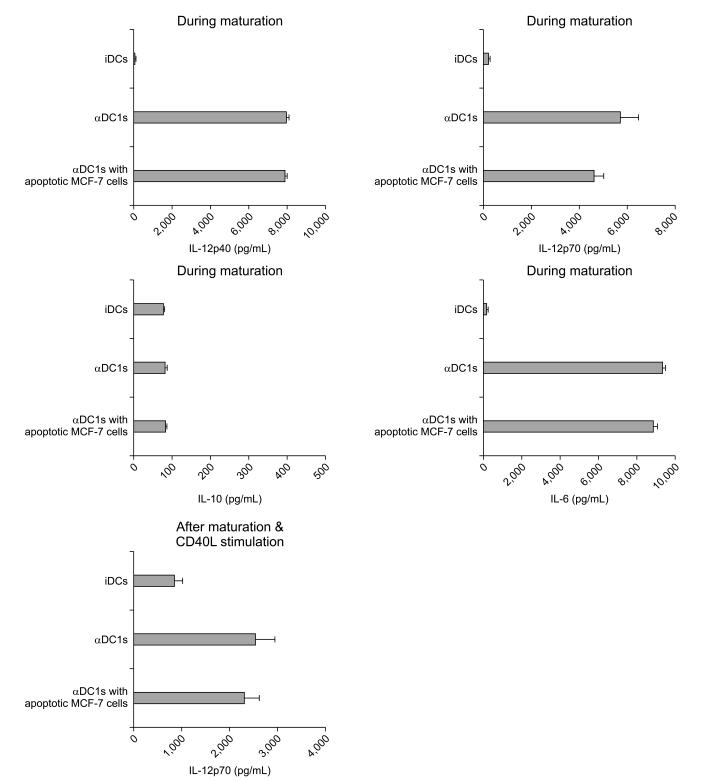 | Fig. 4Comparison of cytokine production by dendritic cells (DCs). Production of interleukin (IL)-12p40, IL-12p70, IL-10 and IL-6 during maturation of the alpha-type 1 polarized DCs (αDC1s) and the αDC1s loaded with apoptotic tumor cells and during re-stimulation with 40L-tranfected J558 cells for 24 hr after maturation. Results, expressed as mean (pg/mL)±standard deviation of triplicate cultures, are from one representative experiment of three. iDCs, immature DCs. |
3. αDC1s in patients with breast cancer promoted Th1 polarization of naïve T cells
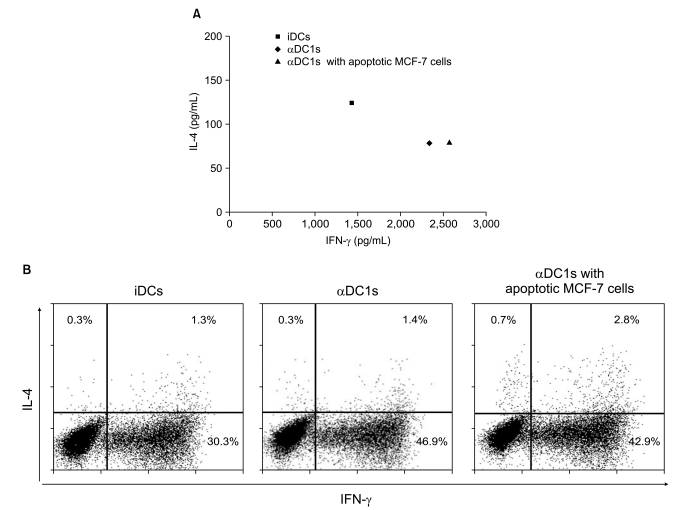 | Fig. 5Alpha-type 1 polarized DCs (αDC1s) induce naïve CD4+ T cells to secrete Th1 cytokines. Allogeneic naïve CD4+CD45RA+ T cells were primed with DCs for 5 days and then stimulated with rIL-2. On day 10, the cells were re-stimulated with Dynabeads CD3/CD28 T cell expander for 1 day. (A) Interleukin (IL)-4 and interferon (IFN)-γ levels measured in culture supernatants by enzyme-linked immunosorbent assay. (B) IL-4 and IFN-γ expression by intracellular staining at the single-cell level. The results are one representative experiment of three independent experiments and are expressed as the percent of cytokine-producing cells. iDCs, immature DCs. |
4. αDC1s in patients with breast cancer showed strong allogeneic T cell stimulatory capacities
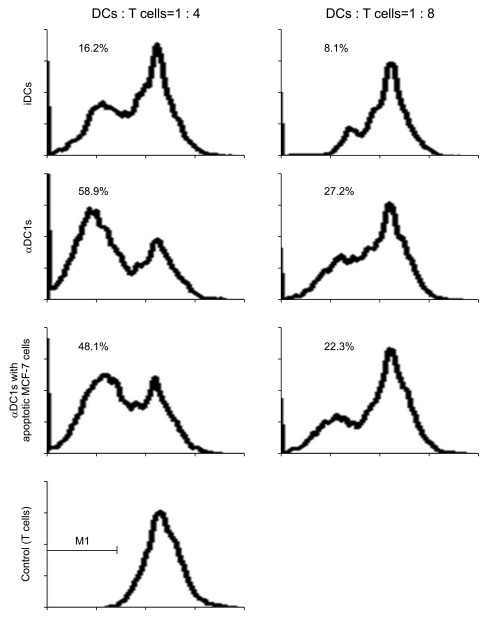 | Fig. 6Comparison of allogeneic T cell stimulatory capacities of dendritic cells (DCs). In a carboxyfluorescein diacetate succinimidyl ester (CFSE)-based proliferation assay, CFSE-labeled allogeneic CD3+ T cells (20,000 cells/well) were stimulated for 5 days with graded doses of irradiated DCs. Flow cytometry was used to measure the percent of CFSE-proliferated cells. The stimulatory capacity of the alpha-type 1 polarized DCs (αDC1s) was significantly higher in a dose-dependent manner than that of the immature DCs (iDCs), and there was no effect on T cell proliferation by loading of apoptotic tumor cells onto αDC1s. Data are representative histograms of fluorescence-activated cell sorting analysis from one of three independent experiments. |
5. Strong breast-cancer-specific CTLs were induced by potent DCs loaded with apoptotic allogeneic breast cancer cells
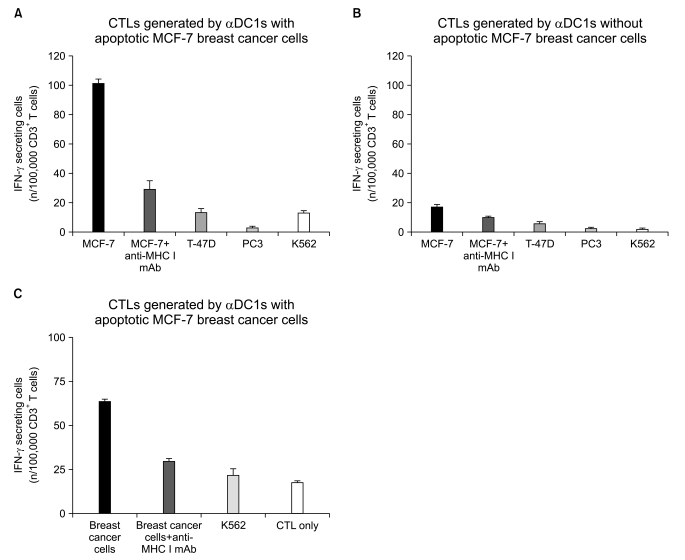 | Fig. 7Ezyme-linked immunospot (ELISPOT) assay to measure the interferon (IFN)-γ release of breast-cancer-specific cytotoxic T lymphocytes (CTLs). Autologous CD3+ T cells were stimulated with dendritic cells (DCs) loaded with apoptotic MCF-7 cells two times every 10 days. On day 20, the CTLs were co-cultured with autologous target cells (MCF-7, HLA-A0201+) and irrelevant target cells (T-47D, HLA-A0201- breast cancer cells; PC3, prostate cancer cells; K562, natural killer cell-sensitive chronic myeloid leukemia cells). (A) Number of IFN-γ-secreting cells from CTLs stimulated with the MCF-7-loaded-alpha-type 1 polarized DCs (αDC1s) (A) and the MCF-7-unloaded-αDC1s (B). Anti-major histocompatibility complex (MHC) class I antibodies were used to confirm the MHC class I-restricted recognition of breast-cancer-specific CTLs. Breast cancer cells were isolated from tumor tissue of patients and the CTLs were co-cultured with the breast cancer cells as target cells (C). The ELISPOT data are the mean (±standard deviation) number of IFN-γ-producing cells of triplicate cultures in three independent experiments. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download