Abstract
Purpose
To evaluate treatment outcomes and prognostic factors in non-small cell lung cancer (NSCLC) patients treated with concurrent chemoradiation.
Materials and Methods
From January 2005 to June 2009, 51 patients were treated with concurrent chemoradiation for 3 different aims: locally advanced stage III, locally recurrent disease, and postoperative gross residual NSCLC. Median age was 63 years. Distribution of stages by the 6th edition of American Joint Committee on Cancer (AJCC) was as follows: IIIA (37.3%), IIIB (56.9%). Chemotherapy was administered every week concurrently with radiation using one of the following regimens: paclitaxel (60 mg/m2), docetaxel+cisplatin (20 mg/m2+20 mg/m2), cisplatin (30 mg/m2). Total radiation dose was 16-66.4 Gy (median, 59.4 Gy).
Results
Median follow-up duration was 40.8 months. The overall response rate was 84.3% with 23 complete responses. The median survival duration for the overall patient group was 17.6 months. The 3-year survival rate was 17.8%. A total of 21 patients had recurrent disease at the following sites: loco-regional sites (23.6%), distant organs (27.5%). In the multivariate analysis of the overall patient group, a clinical tumor response (p=0.002) was the only significant prognostic factor for overall survival (OS). In the multivariate analysis of the definitive chemoradiation arm, the use of consolidation chemotherapy (p=0.022), biologically equivalent dose (BED)10 (p=0.007), and a clinical tumor response (p=0.030) were the significant prognostic factors for OS.The median survival duration of the locally recurrent group and the postoperative gross residual group were 26.4 and 23.9 months, respectively.
Conclusion
Our study demonstrated that clinical tumor response was significantly associated with OS in the overall patient group. Further investigations regarding the optimal radiation dose in the definitive chemoradiation and the optimal treatment scheme in locally recurrent NSCLC would be required.
Despite continuous progress in cancer treatment, lung cancer remains the most common cause of cancer-related deaths in Korea [1] as well as worldwide [2]. Although the overall survival (OS) rate has gradually improved, lung cancer still has a high mortality rate because local and distant failures are common.
Non-small cell lung cancer (NSCLC) is a heterogeneous group of diseases that accounts for about 80%of lung cancer cases. Although surgery can be curative at the early stages of NSCLC, the majority of patients with NSCLC may already be in a locally advanced stage that is not amenable to curative resection at diagnosis.
Historically, thoracic radiotherapy has played a major role in the management of locally advanced NSCLC, and many prospective clinical trials [3-5] have established the benefits of incorporating chemotherapy with radiotherapy over radiotherapy alone. In recent years, the improvement in survival rates has been attributed to the development of modern chemotherapeutic agents and advances in radiation therapy techniques which improved local tumor control without significantly increasing radiation-related morbidity. However, OS and prognosis are still poor in locally advanced NSCLC. There are some variations in the standard of care for patients with locally advanced disease. While surgery can be the treatment of choice for selected N2 positive patients, a neoadjuvant treatment scheme [6] may be used for potentially resectable diseases. Definitive chemoradiation [7,8] may be standard for patients with locally advanced inoperable NSCLC. Other treatment options, such as induction chemotherapy followed by chemoradiation [9], chemoradiation followed by consolidation chemotherapy [10,11] or preoperative chemoradiation followed by surgery [6,12] can be used, but outcomes have not been established.
There are frequently encountered problems such as gross residual diseases after surgical resection, especially in stage III patients, or locally recurrent disease. In patients with a good performance status, aggressive treatment, such as concurrent chemoradiation, could be helpful because we can eradicate residual disease more effectively after incomplete surgery. Some reports have shown that the survival of patients with locally recurrent NSCLC is comparable to that of patients initially diagnosed with locally advanced NSCLC [13]. Furthermore, there has been a study reporting that patients with locally recurrent NSCLC who were treated with curative intent survived much longer than those who were treated with palliative intent [14,15].
In the current study, we retrospectively analyzed treatment results, clinical responses, toxicities, and prognostic factors associated with the OS of patients who received concurrent chemoradiation for locally advanced stage III NSCLC, postoperative gross residual diseases, and locally recurrent NSCLC.
Between January 2005 and June 2009, the Seoul St. Mary's Hospital Lung Cancer Multidisciplinary Treatment Team enrolled 55 patients who were histologically confirmed as having NSCLC. This included locally advanced stage III NSCLC, postoperative gross residual diseases, and locally recurrent NSCLC. Among these 55 patients, we excluded 4 who had not reached the time of clinical response evaluation. All patients had pathologically confirmed measurable disease, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2, and acceptable pulmonary, bone marrow, liver and renal functions. For stage evaluation, patients underwent a chest X-ray, computed tomography (CT) scans of the chest including upper abdomen, bronchoscopy, and positron emission tomography/computed tomography (PET/CT). Tc-99m whole body bone scans, whole abdominal CT scans, and magnetic resonance imaging of the brain were selectively performed when clinically indicated. Mediastinoscopy was not routinely done; it was performed in 6 of 28 patients in the definitive chemoradiation group to discriminate between N2 and N3 diseases.
All patients underwent CT simulation for three-dimensional conformal radiotherapy planning, and radiation was delivered with 6 MV to 15 MV of photon beam energy by linac-based radiation units. We used a radiation therapy planning system (Pinnacle ver. 7.6, ADAC Laboratories, Milpitas, CA) for treatment planning. The total radiation dose was 16-66.4 Gy (median, 59.4 Gy), and the fractional size of 1.8 or 2 Gy was prescribed 5 times a week. The gross tumor volume (GTV) was defined as the primary tumor mass plus the involved lymph nodes. The clinical target volume (CTV) was defined as the GTV plus a 3-D expansion of 0.5-1 cm including the ipsilateral hilum. The planning target volume (PTV) was defined as the CTV plus a 1 cm expansion circumferentially and a 1-2 cm expansion in the superior-inferior directions. We did not treat elective lymph node stations. The entire PTV was encompassed within the 90-95% isodose surface. Dose limits for normal tissue were as follows: spinal cord received≤45-49 Gy (at any point), the lung volume received≥20 Gy (V20)≤25-30%, and a mean lung dose (MLD)≤18-20 Gy. Beam arrangement was planned to minimize the irradiated lung volume usually using 3 to 5 coplanar oblique beams. Some patients having a bulky tumor were treated with an anteroposterior-posteroanterior field to include the CTV for the first 30-40 Gy, followed by off-cord oblique beams usually composed of 3-5 beams. Boost field to GTV with reduced margin was routinely used after 46-50 Gy. Chemotherapy was administered weekly using one of the following regimens: cisplatin (30 mg/m2), paclitaxel (60 mg/m2), docetaxel (20 mg/m2) plus cisplatin (20 mg/m2). When hematologic toxicity was severe during treatment, chemotherapy administration was delayed based on the medical oncologist's decision.
Consolidation chemotherapy was administered 4 weeks after the finish of the chemoradiation course by the following regimens: 2-4 cycles of docetaxel and cisplatin in 8 patients, 4 cycles of paclitaxel and cisplatin in 4 patients, and 1 cycle of gemcitabine and carboplatin in 1 patient.
Blood chemistry and a complete blood count were obtained weekly, or more frequently, if needed during the treatment periods. We performed chest CT and/or PET/CT for the evaluation of treatment responses between 4 and 12 weeks after the end of the chemoradiation treatment course. The World Health Organization (WHO) criteria were used for response evaluations. These consisted of complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). Acute toxicity was assessed using the National Cancer Institute common toxicity criteria (NCI CTCAE) ver. 3.
OS was defined as the time from the histologically confirmed date to the date of death or to the patient's last visit. Progression free survival (PFS) was defined as the time from the histologically confirmed date to the date of disease progression or the date of the patient's last visit. In case of locally recurrent disease, a starting point for the calculation of OS or PFS was the date on which recurrence was confirmed by serial radiologic findings, or histologically if specimens were available. For statistical analysis, SPSS ver.12.0 (SPSS Inc., Chicago, IL) was used. Kaplan-Meier methods were used to calculate OS and PFS. A log-rank test was used to compare survival differences between treatment groups, and the Cox proportional hazards regression model was used to identify independent prognostic factors and to determine the impact of factors on OS. All p-values were calculated based on a two-sided test.
The number of patients in each treatment group was as follows: 28 in locally advanced stage III NSCLC, 11 in postoperative gross residual disease, and 12 in locally recurrent NSCLC. Patient characteristics are shown in Table 1. The median age was 63 years (range, 40 to 79 years), and 82.4% of patients were male. The majority of patients had an ECOG PS score of 0-1 (92.1%), and the distribution of histology findings were squamous cell carcinoma (54.9%) and adenocarcinoma (33.3%). Stage was determined by the 6th edition of the American Joint Committee on Cancer (AJCC) staging system. If pathologic specimens were available, we used pathologic stages for tumor-node-metastasis (TNM) staging. The distribution of clinical stages was as follows: IIIA (37.3%), IIIB (56.9%).
Four patients were treated with radiation doses of less than 50 Gy, which was inappropriate for achieving an optimal radiation effect. In one patient, treatment was discontinued after 16 Gy of radiotherapy because acute dyspnea developed after 2 cycles of concurrent chemoradiation. These symptoms were probably due to a hypersensitivity reaction to the chemotherapeutic agent. In another patient, treatment was discontinued after 42 Gy of radiotherapy because abrupt pneumothorax developed during treatment. In the remaining 2 patients, treatment-related toxicities were not severe, but the treatment was discontinued after their respective radiation doses of 32.4 Gy and 46.8 Gy by patient decision because of poor general conditions or other reasons. The median number of chemotherapy cycles during the concurrent chemoradiation treatment was 6 (range, 2-8). Forty-five patients (88.2%) were treated with at least 6 cycles of chemotherapy.
Response evaluation was done by chest CT with or without PET/CT. The number of patients with a response evaluation at 1 month after the end of chemoradiation was 8. The other patients were evaluated at 3 months after the end of chemoradiation. The overall response rate (CR+PR) was 84.3% (n=43), including 23 CR (45.1%). Clinical response in each treatment group is described in Table 2. The postoperative gross residual group showed a relatively high CR rate (81.8%), probably due to the reduced tumor burden after surgical resection. There was no difference in the overall response rates among different chemotherapeutic regimens (p=0.194).
At the time of this analysis, recurrence has been observed in 21 patients (41%). The distribution of the first site of recurrence is shown in Fig. 1. Tumors recurred in the following sites: locoregional sites (23.6%) and distant organs (27.5%). The most common site of distant metastasis was the brain followed by skeletal system. All of the patients with recurrent disease received palliative treatments including chemotherapy, molecular targeted therapy or localized radiotherapy.
The median follow-up duration in the entire population was 40.8 months (range, 3 to 69.9 months). The median PFS in the entire population was 12.5 months, and the 1-year and 2-year PFS rates were 51% and 23%, respectively. There was no statistically significant difference between treatment groups (p=0.583) (Figs. 2 and 3). The median OS in the entire population was 17.6 months, and the 2-year and 3-year OS rates were 42% and 17.8%, respectively. Median survival was different between treatment groups but not statistically significant (p=0.638) (Figs. 2 and 3).
The results of univariate and multivariate analysis affecting OS in the entire population are shown in Table 3. The covariables were gender, age, histologic type, ECOG PS score, number of chemotherapeutic agents, chemotherapeutic regimen, clinical tumor response, T stage, N stage, clinical stage and tumor size. In the univariate analysis, a better ECOG PS score (0 vs. 1-2, p=0.042) and higher clinical tumor response (CR+PR vs. SD+PD, p=0.002) were significantly associated with improved OS, and a trend was detected that patients with<65 years had better survival than those with≥65 years (p=0.062). Among these factors, clinical tumor response (p=0.002) was the only independent factor associated with improved OS in the multivariate analysis. Kaplan-Meier OS curves stratified by clinical tumor response in the overall patients group is shown in Fig. 4.
We performed subgroup analysis of the definitive chemoradiation arm to evaluate prognostic factors. This analysis included age, gender, histologic type, ECOG PS score, number of chemotherapeutic agents, chemotherapeutic regimen, T stage, N stage, clinical stage, tumor size, use of consolidation chemotherapy, total radiation dose, which was converted into biologically equivalent dose (BED), and clinical tumor response. Among these factors, the use of consolidation chemotherapy (p=0.042), BED10≥70 (p=0.011) and higher clinical response (p=0.0049) were significantly associated with improved OS in the univariate analysis.
In the multivariate analysis, the use of consolidation chemotherapy (p=0.022), BED10≥70 (p=0.007), and higher clinical tumor response (p=0.030) were the independent prognostic factors associated with improved OS. The Results of univariate and multivariate analyses of the definitive chemoradiation arm are shown in Table 4 and Kaplan-Meier OS curves stratified by clinical tumor response, BED10 and use of consolidation chemotherapy are shown in Fig. 5.
Hematologic and esophageal toxicity were major acute toxicities. The incidence of neutropenia (≥grade 3) and esophagitis (≥grade 3) were 31.4% and 27.5%, respectively. Radiation pneumonitis (≥grade 3) occurred in 2 patients. MLD of these two patients was 8.8 Gy and 14.8 Gy, respectively. The volume of lung that received ≥20 Gy (V20) were 31% and 41%, and ≥30 Gy (V30) were 24% and 32%, respectively. There were 2 toxic deaths associated with acute radiation pneumonitis. One death was caused by viral pneumonia superimposed on acute radiation pneumonitis during hospitalization around 1 month after the end of the treatment. Another patient died of acute respiratory distress syndrome during hospitalization 3 months after the end of treatment. Acute toxicities of the treatment are listed in Table 5. Chronic esophageal toxicities were observed in 2 patients who presented with esophageal stricture and fistula, respectively.
Before the 1990s, locally advanced NSCLC was treated with radiation alone. After many randomized trials revealed that the integration of systemic chemotherapy with radiotherapy would be more beneficial than radiotherapy alone [3-5], combination chemoradiation, especially concurrent chemoradiation rather than sequential chemoradiation [8,16,17], has become one of the standard treatments. Recently, the Intergroup 0139 [12] study showed improved survival with preoperative chemoradiation followed by surgery over definitive chemoradiation in IIIA-N2 NSCLC when lobectomy was possible. This neoadjuvant chemoradiation protocol can be used to treat selected minimal N2 NSCLC patients who become resectable. However, additional trials with more population-based studies would be required to show conclusive results. In the current study, we analyzed the treatment results of 3 different groups: locally advanced stage III NSCLC, postoperative gross residual disease, and locally recurrent NSCLC. The median OS time for all patients was 17.6 months, and the 2-year and 3-year OS rates were 42% and 17.8%, respectively. Those treatment outcomes are comparable with previous reports [7-11,18]. Among the different groups, locally advanced NSCLC (n=28, 54.9%) was the most common. The clinical development of third generation chemotherapy showed potent radiosensitization effects, and those chemotherapeutic agents are commonly used with radiotherapy in the definitive chemoradiation setting [13,19]. The proportion of patients in our study receiving combined taxane chemotherapy (paclitaxel or decetaxel) was 82.4%, which was a very high frequency. However, differences in a number or regimen of chemotherapeutic agent (single vs. double, taxane vs. non-taxane, platinum vs. non-platinum) did not affect OS significantly (Table 3). Our study showed different median survival durations between treatment groups: 17.6 months in full analysis patients, 13.2 months in the definitive chemoradiation group, 26.4 months in the locally recurrent group, and 23.9 months in the postoperative gross residual group. In the definitive chemoradiation group, median OS duration was relatively shorter than that of previously reported. This was probably due to the fact that most of the patients enrolled in our study were in stage IIIB (71.4%), and a few of those patients had shorter follow up periods. We compared the effect of the total radiation dose on OS between two groups of patients-9 patients who received 66 Gy and another 12 patients who were treated with 59-60 Gy. However, we found that there was no significant difference in OS between the two groups. This lack of significance might be due to the small sample size of the group or the narrow range of total radiation dose differences between the two groups. Therefore, re-evaluation with longer follow up of larger sample sizes will be required for more definitive conclusions. In the locally recurrent group, we noted reliably longer OS with a non-inferior outcome when compared with the initially diagnosed locally advanced NSCLC group. This was supported by the several previous studies [13,14], which implies that aggressive treatment such as concurrent chemoradiation or dose escalation radiotherapy would result in improved survival in locally recurrent cases. Among 12 locally recurrent patients in our study, two had only bronchial stump recurrence and ten had mediastinal lymph node recurrence with or without bronchial stump recurrence. There have been a few studies reporting that bronchial stump recurrence has better prognosis than cases combined with mediastinal lymph node recurrence or chest wall invasion. Foo et al. [15] reported that patients treated with radical intent survived much longer. The median survival was 26 months in the radical intent group and 10.5 months in the palliative intent group. Although we included the majority of mediastinal lymph node recurrences, our patients had relatively long OS durations. Our concurrent chemoradiation scheme might contribute to this outcome, and this scheme could be justified for locally recurrent cases, especially those with mediastinal lymph node recurrences. Further studies are now required to define the beneficial role of combining chemotherapy with radiotherapy in loco-regionally recurrent NSCLC.
Treatment toxicity was acceptable when compared with previous studies of concurrent chemoradiation treatment. However, there were 2 toxic deaths associated with radiation pneumonitis, 1-3 months after completion of chemoirradiation in 2 elderly patients, which suggests that a careful evaluation of pulmonary function and close follow up will be needed after treatment in an immune-suppressed host.
In the analysis of prognostic factors, the clinical tumor response was significantly associated with OS not only in the entire patient cohort, but also in the definitive chemoradiation group. However, we could not find any significant factors associated with good responses such as total radiation dose, chemotherapeutic regimen, TNM stage or histologic type. From the fact that the reduced tumor burden after surgery resulted in a relatively high CR rate (81.8%), we can only infer that the tumor burden (tumor volume) could be a significant factor. In our study, the T or N stage was not associated with the response rate, and this may be due to a small sample size. There have been significant advances in molecular biology, and these will help pinpoint the factors associated with responses to therapy.
There are several known pre-treatment prognostic factors associated with OS in NSCLC such as weight loss, tumor stage, performance status and pulmonary function [20,21]. We couldn't examine all of these factors due to the limitations of a retrospective study. However, among the pre-treatment factors, ECOG PS was the most strongly associated with OS.
In subgroup analysis of the definitive chemoradiation group, the use of consolidation chemotherapy, BED10≥70, and higher clinical tumor response were the independent prognostic factors that improved OS. Furthermore, our subgroup analysis of locally recurrent and postoperative gross residual diseases indicated that clinical tumor response and PS are significant prognostic factors affecting OS (data not shown). This means that more radical tumor control will eventually be connected to improved survival in NSCLC.
In the SWOG 9504 [10] trial, concurrent chemoradiation with etoposide-cisplatin followed by consolidation docetaxel were administered to stage IIIB patients. The result was encouraging with a median survival time of 26 months, and 3-year and 5-year survival rate were 37% and 29%, respectively. After the SWOG 9504 trial, several phase III randomized trials [11,22,23] were conducted to define the role of consolidation chemotherapy in locally advanced NSCLC. However, different results were shown among the studies, and some studies showed a relatively high frequency of high-grade hematologic and esophageal toxicities. In the most recently reported interim analysis of a multinational phase III randomized trial (CCheIN) [23], consolidation chemotherapy with docetaxel plus cisplatin (DP) after concurrent chemoradiation (CCRT) with weekly DP was shown to be feasible and tolerable, but there were no statistically significant differences in time to progression and median OS between observation and consolidation arms. As a result, further investigations are needed to precisely define the role of consolidation chemotherapy after CCRT in locally advanced NSCLC. We would cautiously infer that stage IIIB patients with good PS will benefit mostly from the consolidation chemotherapy. In the same context, our study showed that the benefit of consolidation chemotherapy was probably due to the high proportion of IIIB patients in the definitive chemoradiation arm.
To further evaluate the radiation dose effect, we calculated BED using an α/βratio of 10 because the fractional size was different. However, some patients treated with a less than optimal radiation dose were included in the study, and we did not apply a time factor in calculating BED. Thus, a follow up study including a larger cohort should be performed. In our study, patients treated with BED10≥70 showed a better OS when compared to those with BED10≥70, and this was the independent prognostic factor associated with improved OS in the multivariate analysis (hazard ratio, 6.184; 95% confidence interval, 1.644 to 23.259; p=0.007). This result needs careful interpretation: it should be interpreted as the optimal minimum dose to get the radiation effect attributed to improving overall survival, not the appropriate radiation dose in the CCRT setting. Many clinical trials have been performed with a radiation dose of 60-74 Gy [11,23], and the optimal radiation dose in concurrent chemoradiation setting remains unclear. Although some studies showed that a higher radiation dose may be correlated with improved local control and OS [24], the results of the ongoing phase III intergroup study (RTOG 0617) (60 Gy vs. 74 Gy) will more definitively identify the additional role of a higher radiation dose in the chemoradiation setting.
The current study demonstrated that ECOG PS and clinical tumor response were the independent prognostic factors despite heterogeneous composition of the subgroups. In subgroup analysis of the definitive chemoradiation group, the use of consolidation chemotherapy, BED10≥70 and higher clinical tumor responses were the independent prognostic factors correlated with the improved OS. In locally recurrent NSCLC, our study showed the relatively better OS outcomes compared with previous studies. However, our study needs a longer follow up with more population group for more precise outcomes. Further investigations would be required to define the factors associated with improving tumor response, the role of optimal radiation dose in definitive chemoradiation setting, and the optimal treatment scheme in locally recurrent NSCLC.
References
1. Korea Central Cancer Registry. Annual report of causes of deaths, 2009. 2010. Seoul: Ministry of Health and Welfare.
2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96. PMID: 18287387.

3. Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990; 323:940–945. PMID: 2169587.

4. Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Tarayre M, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991; 83:417–423. PMID: 1847977.

5. Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996; 88:1210–1215. PMID: 8780630.

6. Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol. 2007; 25:313–318. PMID: 17235046.

7. Albain KS, Crowley JJ, Turrisi AT 3rd, Gandara DR, Farrar WB, Clark JI, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002; 20:3454–3460. PMID: 12177106.

8. Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004; 46:87–98. PMID: 15364136.

9. Socinski MA, Morris DE, Halle JS, Moore DT, Hensing TA, Limentani SA, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004; 22:4341–4350. PMID: 15514375.

10. Gandara DR, Chansky K, Albain KS, Leigh BR, Gaspar LE, Lara PN Jr, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003; 21:2004–2010. PMID: 12743155.

11. Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008; 26:5755–5760. PMID: 19001323.

12. Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009; 374:379–386. PMID: 19632716.

13. Leung J, Ball D, Worotniuk T, Laidlaw C. Survival following radiotherapy for post-surgical locoregional recurrence of non-small cell lung cancer. Lung Cancer. 1995; 13:121–127. PMID: 8581391.

14. Kelsey CR, Clough RW, Marks LB. Local recurrence following initial resection of NSCLC: salvage is possible with radiation therapy. Cancer J. 2006; 12:283–288. PMID: 16925972.
15. Foo K, Gebski V, Yeghiaian-Alvandi R, Foroudi F, Cakir B. Outcome following radiotherapy for loco-regionally recurrent non-small cell lung cancer. Australas Radiol. 2005; 49:108–112. PMID: 15845045.
16. Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999; 17:2692–2699. PMID: 10561343.

17. Fournel P, Robinet G, Thomas P, Souquet PJ, Léna H, Vergnenégre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol. 2005; 23:5910–5917. PMID: 16087956.

18. Ahn SJ, Kim YC, Kim KS, Park KO, Chung WK, Nam TK, et al. Results of curative radiation therapy with or without chemotherapy for stage III unresectable non-small cell lung cancer. Cancer Res Treat. 2005; 37:268–272. PMID: 19956525.

19. Mudad R, Ramsey M, Kovitz K, Curiel TJ, Hartz R, Nedzi LL, et al. Concomitant weekly docetaxel, cisplatin and radiation therapy in locally advanced non-small cell lung cancer: a dose finding study. Lung Cancer. 2003; 39:173–177. PMID: 12581570.

20. Firat S, Byhardt RW, Gore E. Radiation Therapy Oncology Group. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Int J Radiat Oncol Biol Phys. 2002; 54:357–364. PMID: 12243808.

21. Ademuyiwa FO, Johnson CS, White AS, Breen TE, Harvey J, Neubauer M, et al. Prognostic factors in stage III non-small-cell lung cancer. Clin Lung Cancer. 2007; 8:478–482. PMID: 17922971.

22. Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008; 26:2450–2456. PMID: 18378568.

23. Park K, Ahn Y, Chen M, Cho E, Kim J, Min Y, et al. A multinational phase III randomized trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small cell lung cancer (CCheIN): interim analysis. J Clin Oncol. 2009; 27(15s):7538.

24. Rengan R, Rosenzweig KE, Venkatraman E, Koutcher LA, Fox JL, Nayak R, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004; 60:741–747. PMID: 15465190.

Fig. 1
First site of recurrence. The patterns of failure were locoregional relapse in 6 (11.8%) patients, distant metastasis in 8 (15.7%) patients, and combined loco-regional and distant failure in 6 (11.8%) patients.
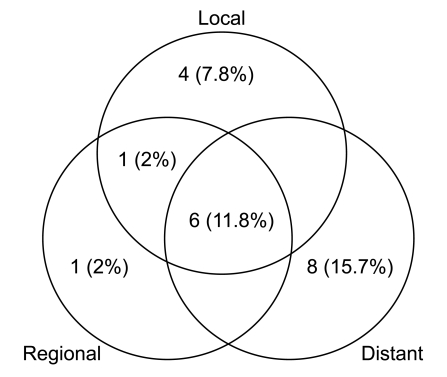
Fig. 2
Overall survival (A) and progression free survival (B) curves for the overall patient group. The 2-yr and 3-yr overall survival rates were 42% and 17.8%, respectively. The 1-yr and 2-yr progression free survival rates were 51%and 23%, respectively.
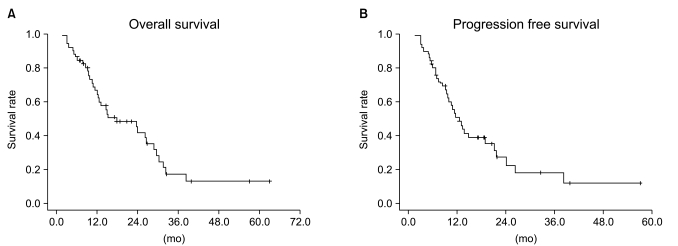
Fig. 3
Overall survival (A) and progression free survival (B) curves according to the treatment arms. There was no statistical difference between the treatment groups with regard to overall survival (p=0.6389) and progression-free survival (p=0.5833).
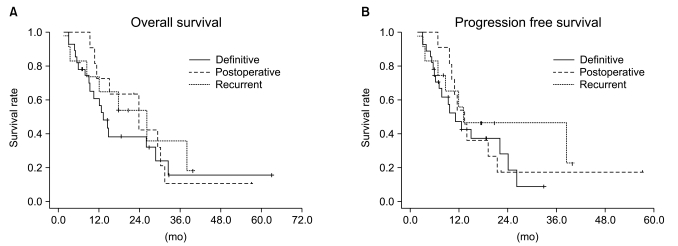
Fig. 4
Overall survival curves stratified by clinical tumor response for the total patient group. Overall survival rate between responder (complete response+partial response [CR+PR]) and non-responder (stable disease+progressive disease [SD+PD]) groups was statistically different on log-rank test (p=0.002).
Fig. 5
Overall survival curves stratified by (A) clinical tumor response, (B) biologically equivalent dose (BED)10, (C) use of consolidation chemotherapy in the definitive arm. The difference in overall survival rate between responder (complete response+partial response [CR+PR]) and non-responder (stable disease+progressive disease [SD+PD]), BED10≥70 and BED10<70, was statistically different, as was the difference between use of consolidation chemotherapy and no use of consolidation chemotherapy (p=0.049, 0.011 and 0.042, respectively) on log-rank test for the definitive arm.
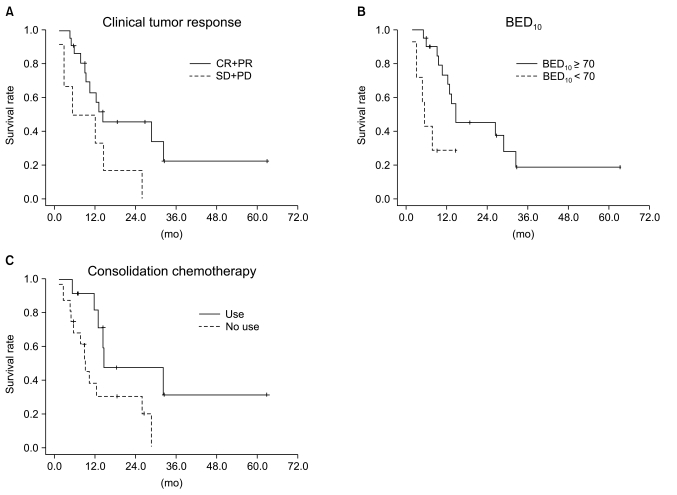
Table 1
Patient, tumor and treatment characteristics in the overall patient group
Table 2
Clinical response rate in the overall patient and each treatment arms
| Clinical response | Responder (%) | Non-responder (%) | Median survival (mo) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| CR | PR | SD | PD | Responder | Non-responder | ||
| Overall | 23 (45.1) | 20 (39.2) | 6 (11.8) | 2 (3.9) | p=0.054a) | 23.9 | 8.4 |
| Definitive | 7 (25) | 15 (53.6) | 4 (14.3) | 2 (7.1) | 14.6 | 5.4 | |
| Postoperative | 9 (81.8) | 1 (9.1) | 1 (9.1) | 23.9 | 10.6 | ||
| Recurrent | 7 (58) | 4 (33.3) | 1 (8.3) | 26.4 | 8.4 | ||
Table 3
Univariate and multivariate analysis for the overall patient group
Table 4
Univariate and multivariate analysis of the definitive arm




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download