Abstract
Purpose
The pharmacological activities, notably the anticancer properties, of bioactive constituents fromfresh American ginseng berry have not yet been well studied. In this study, we investigated the antiproliferative effects of fresh American ginseng berry extract (AGBE) and its representative triterpenoid glycosides using the human colorectal cancer cell line SW480.
Materials and Methods
Using high performance liquid chromatography (HPLC), the contents of 8 ginsenosides in AGBE were determined. The cell growth inhibitory effects of AGBE and three triterpenoid glycosides (ginsenosides Rb3, Re, and Rg3) were evaluated by proliferation assay and 3H-thymidine incorporation assay. Cell cycle and apoptotic effects were analyzed by using flow cytometry after staining with propidium iodide and annexin V.
Results
HPLC analysis data showed that AGBE has a distinct ginsenoside profile. AGBE inhibited SW480 cell growth significantly in a time-dependent (24-96 hours) and concentration-dependent (0.1-1.0 mg/mL) manner. Ginsenosides Rb3, Re, and Rg3 also possess significant antiproliferative activities on SW480 cells. 3H-thymidine incorporation assay indicated that AGBE and ginsenosides Rb3, Re, and Rg3 might inhibit the transferring and duplication of DNA in SW480 cells. Flow cytometric assay data suggested that AGBE arrested SW480 cells in S and G2/M phases, and significantly induced cell apoptosis.
Human colorectal cancer is a significant public health problem in the western world. In the United States, this cancer is the third leading cause of cancer related death and the third most prevalent cancer worldwide. Half of all patients diagnosed with colorectal cancer will eventually die from the disease; and less than 10% of patients with metastatic colorectal cancer will survive more than five years after diagnosis. The American Cancer Society estimates that 106,100 new cases of colon cancer (52,010 men and 54,090 women) and 40,870 new cases of rectal cancer (23,580 men and 17,290 women) were diagnosed in 2009 [1]. It has also been estimated that in the United States colorectal cancer caused about 10% of all cancer-related deaths during 2009 with 49,920 deaths (25,240 men and 24,680 women) [1]. Over 90% of colon cancers are adenocarcinomas developing from the glandular crypts lining the large bowel. More interestingly, previous reports also indicated that up to 80% of all colorectal cancer cases and deaths are attributable to diet [2] and such cases of colorectal cancer and related deaths may be prevented by dietary modifications. Although many early stage colorectal cancers are cured by surgical resection alone, surgery is most often combined with adjuvant radio- and chemotherapy. Radiotherapy is often combined with one or more chemotherapeutic agents. Patients treated for advanced staged colorectal cancer with adjuvant chemotherapy showed a 40% reduced risk of relapse [3,4]. On the other hand, the great majority of studies showed that diet with adequate or high levels of fruits and vegetables reduced risk for the disease. The preponderance of the evidence favors the hypothesis that natural compounds in botanicals protect against the development of colon cancer [5,6]. Thus, there is a need to look for new herbal medicines and evaluate their chemical compounds to protect against the development of colon cancer, or look for complementary and alternative medicines to treat or reduce risk of relapse.
Asian ginseng (Panax ginseng) has been a valuable herbal medicine used in Oriental countries for thousands of years [7-9]. American ginseng (P. quinquefolius) has gained popularity with the American public over the past decade [7,10]. Previous studies have demonstrated that ginseng root and its major bioactive components (ginsenosides) have complex constitutions and multifaceted pharmacological functions, such as anticancer properties [11,12]. The known biochemical and pharmacological activities of ginseng include antiaging, antidiabetic, anticarcinogenic, analgesic, antipyretic, antistress, antifatigue, and tranquilizing properties as well as the promotion of DNA, RNA, and protein synthesis [7,13,14]. Most of the reports, however, have focused upon the root of ginseng for the various functions, including anticancer activities. There is very limited data available in the literature with regard to cancer chemoprevention of fresh American ginseng berry extract (AGBE) and its triterpenoid glycosides, commonly referred to ginsenosides [15]. Therefore, the aim of the present study was to explore further the antiproliferative property of AGBE and ginsenosides, Re, Rb3, and Rg3, the single compounds in AGBE, in human colorectal cancer cells.
Fresh American ginseng berry was collected from a private ginseng farm in Wausau, WI. The fresh berry was mixed with organic solvent. After removing the seeds and the pulp, the organic solvent was evaporated under a vacuum. Finally, the condensed extract solution was lyophilized.
HPLC analysis was conducted on a Waters HPLC system, with a 996 photodiode array detector (Milford, MA). The separation was carried out on a Prodigy™ ODS [2] column (150×4.6 mm I.D.; Phenomenex, Torrance, CA) with a guard column (3.0×4.0 mm I.D.; Prodigy™, Phenomenex). For HPLC analysis, a 20-µL sample was injected into the columns and eluted at room temperature with a constant flow rate of 1.0 mL/min. Acetonitrile (solvent A) and water (solvent B) were used. The gradient elution started with 20% solvent A and 80% solvent B; changed to 20.3% A for 25 minutes; then changed to 26.8% A for 3 minutes and held for 26 minutes; changed to 35.6% A for 20 minutes; changed to 50% A for 11 minutes; changed to 68% A for 10 minutes; changed to 95% A for 1 minute and held for 3 minutes; changed to 20% A for 3 minutes and held for 8 minutes. The detection wavelength was set to 202 nm. Ginsenosides Rb1, Rc, Rd, Re, and Rg1 were purchased from Indofine Chemical Company (Somerville, NJ); ginsenoside Rb2, Rb3, and Rg3 were purchased from Delta Information Center for Natural Organic Compounds (Xuancheng, China). Regression equations of ginsenosides were prepared using standard solutions with different concentrations.
SW480 human colorectal cells (ATCC, Manassas, VA) were routinely grown in Leibovitz's L-15 medium with 10% L-glutamine (Cellgro, Herndon, VA) and supplemented with 10% fetal bovine serum and 50 units/mL penicillin-streptomycin (Gibco, Carlsbad, CA). Cells were maintained in a tissue culture dish (100 mm I.D.) and kept in a humidified incubator (5% CO2 in air at 37℃) with a medium change every 2-3 days. When the cells reached 70-80% confluence, they were trypsinized, harvested, and seeded into a new tissue culture dish.
To examine the cell growth inhibitory effect of AGBE and ginsenosides, we conducted cell proliferative assay using cell counting method. SW480 cells were seeded in a 24-well plate at approximately 1×104 cells/well with regular Leibovitz's L-15 medium. After adhesion for 24 hours, fresh culture medium was exchanged and then drugs were added. Control cultures were incubated in medium containing solvent vehicle alone. At the end of the drug exposure period, the medium was removed from all wells and the cell monolayer was washed twice with phosphate buffered saline (PBS). Cultures were harvested and monitored for number by using a Coulter cell counter (Coulter Electronics Inc., Hialeah, FL). In the present studies, cell proliferative assays were performed at least three times. The percentage of cell proliferation was calculated as follows: Cell proliferation (%)=(cell number in each drug-treated well/average of cell number in the control wells)×100.
To observe the cell morphological changes induced by AGBE, the SW480 cells were seeded in a 24-well plate with regular Leibovitz's L-15 medium and allowed to adhere for 24 hours. After the SW480 cells incubated with AGBE at 1.0 mg/mL for 72 hours, the cellular morphological changes were observed under phase contrast microscopy (Eclipse TE2000-U, Nikon, Tokyo, Japan). Control cultures were incubated in medium containing solvent vehicle alone.
To explore the possible mechanism of AGBE and its ginsenosides' antiproliferative properties, 3H-thymidine incorporation assay on SW480 cells was performed in the present experiments. The SW480 cells were seeded in a 24-well plate and adhered for 24 hours, the cells were incubated with AGBE, or ginsenosides Rb3, Re, and Rg3, at various concentrations with 300 µL media containing 1 λ/mL 3H-thymidine in each well for 72 hours. Control cultures were incubated in medium containing solvent vehicle alone. Then, cells were washed with PBS and 10% trichloroacetic acid and 0.5 mL NaOH (0.2 M) were added into each well and agitated for 5 minutes. Then, cells were transferred into tested vials, 5 mL of 30% liquid scintillation were added, and radioactivity counts were measured using Packard Tri-Carb 1500 scintillation counter (Meriden, CT). Results are presented as the percentage of control.
Cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed and cells were treated with AGBE. Cells were incubated for 48 hours before they were harvested. For the cell cycle assay, the cells were fixed gently by putting 80% ethanol in a freezer for 2 hours and subsequently treating with 0.25% Triton X-100 for 5 minutes on ice bath. Cells were resuspended in 300 µL of PBS containing 40 µg/mL propidium iodide and 0.1 mg/mL RNase. Then the cells were incubated in a dark room for 20 minutes at room temperature, and cell cycle analysis was performed. For the apoptotic assay, cells floating in the medium were collected after they were treated with AGBE for 48 hours. The adherent cells were detached with trypsin. Then, culture medium containing 10% fetal bovine serum (and floating cells) was added to inactivate trypsin, and pipetted gently. The cells were centrifuged and then stained with annexin V-FITC. Cells were analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and FlowJo ver. 7.1.0 software (Tree Star, Ashland, OR). For each measurement at least 10,000 cells were counted.
HPLC analysis showed that the main constituents in AGBE were ginsenosides Rb3 (49.5 mg/g) and Re (15.1 mg/g). Other ginsenosides contents in AGBE were ginsenosides Rb1 (1.1 mg/g), Rb2 (13.8 mg/g), Rc (5.6 mg/g), Rd (9.0 mg/g), Rg1 (0.8 mg/g), and Rg3 (0.9 mg/g). Fig. 1 shows the chromatogram of ginsenoside standards and AGBE. The proportions of ginsenosides of AGBE were much different with American ginseng root extracts [16,17]. In American ginseng root extract, the highest content of triterpenoid glycoside is ginsenoside Rb1, while ginsenoside Rb3 is a trace triterpenoid glycoside. However, in AGBE, ginsenoside Rb3 has the highest content while the content of Rb1 is much lower. The specific pharmacological effects of AGBE may have originated from the composition of chemical compounds, especially the ginsenosides.
The antiproliferative effects of AGBE on SW480 cells were evaluated. Data showed that AGBE inhibited the growth of SW480 cells in a time-dependent manner. As shown in Fig. 2, AGBE at a single concentration of 1.0 mg/mL decreased the viable number of SW480 cells significantly. Compared to the vehicle control in 24, 48, 72, and 96 hours (set as 100%), the percentage of viable cells was 89.2 at 24 hours (p<0.05), 75.0 at 48 hours (p<0.01), 29.0 at 72 hours (p<0.01), and 3.2 at 96 hours (p<0.01). It is clear that AGBE inhibited SW480 cancer cells in a time-dependent manner.
The growth inhibitory effect of AGBE (0.1, 0.5 and 1.0 mg/mL) against SW480 human colorectal cancer cells is shown in Fig. 3A. After treatment for 72 hours, AGBE decreased viable cell number significantly. The extract reduced cell growth in a concentration-dependent manner, by 16.9% at 0.1 mg/mL (p<0.05), 58.3% at 0.5 mg/mL (p<0.01), and 71.0% at 1.0 mg/mL (p<0.01), compared with control group (normalized to 100%). The data showed inhibition of AGBE on SW480 cancer cells in a dosage-dependent manner in this experiment.
Morphological appearance of SW480 cells under AGBE treatment for 72 hours is shown in Fig. 3B. Exposure of SW480 cells to AGBE had a marked effect on morphology compared to the untreated control cells. In the control group, SW480 cells showed a cobblestone-like appearance, while the cells treated with 1.0 mg/mL of AGBE showed an elongated fibroblastic appearance with many apoptotic bodies. There was a remarkable increase in the number of shrunken cells after 1.0 mg/mL of AGBE treatment. Similar but less severe effects on cell morphological changes were observed with treatment of AGBE in lower concentrations (0.1 and 0.5 mg/mL). These data indicate that AGBE induces prominent changes in the morphological appearances of SW480 cancer cells.
The antiproliferative effects of ginsenosides Re, Rb3, and Rg3 on colon cancer cells SW480 were determined by cell proliferation assay. Similar to the activities of AGBE, ginsenosides Re, Rb3, and Rg3 significantly inhibited SW480 cell growth with a concentration-dependent manner (Fig. 4). Re inhibited cell growth by 16.0% (0.03 mg/mL, p<0.05), 23.0% (0.1 mg/mL, p<0.01), and 39.0% (0.3 mg/mL, p<0.01) compared with vehicle control (100%). The antiproliferative effect of Rb3 is similar to Re. However, Rg3 has inhibition that is more efficient on SW480 cell growth at 0.1 and 0.3 mg/mL. For instance, Rg3 reduced cell proliferation by 6.3% (0.03 mg/mL, p>0.05), 47.3% (0.1 mg/mL, p<0.01), and 94.6% (0.3 mg/mL, p<0.01) compared with the vehicle control (100%). Data from this study demonstrate that ginsenosides Re, Rb3, and Rg3 possess antiproliferative effects on SW480 human colorectal cancer cells.
As shown in Fig. 5A, AGBE (0.5 and 1.0 mg/mL) significantly suppressed cellular incorporation of 3H-thymidine at the concentrations of 0.5 mg/mL (49.0%) and 1.0 mg/mL (by 78.0%, both p<0.01, compared with control). Similar results were obtained from ginsenosides Re, Rb3, and Rg3 (Fig. 5B). Compared to the control (100%), ginsenoside Rg3 suppressed 3H-thymidine incorporation for 26.9% at 0.03 mg/mL (p<0.01), 61.7% at 0.1 mg/mL (p<0.01), and 98.2% at 0.3 mg/mL (p<0.01). Ginsenoside Rg3 reduced the percentage of the incorporation of cells significantly. Similar but lower effects were obtained from Re and Rb3 (Fig. 5B). These results suggest that ginsenosides Re, Rb3, and Rg3 are endowed with inhibiting cellular incorporation of 3H-thymidine, and that Rg3 possesses more positive effects on SW480 cells.
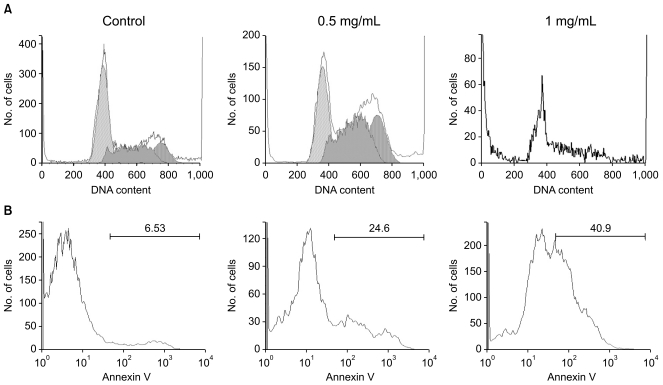
To explore the potential mechanism through which AGBE inhibits cell growth, cell cycle and apoptosis were assayed by flow cytometry. As shown in Fig. 6A, compared to control (G1, 51.4%; S, 32.3%; G2/M, 16.2%), treatment with 0.5 mg/mL of AGBE for 48 hours, 32.3% cells were in G1 phase, 42.9% cells were in S phase, and 24.8% cells were in G2/M phase. Since the viable cells were difficult to collect after treatment with 1 mg/mL of AGBE for 48 hours, the cell cycle profile was not obtained. Nevertheless, data from this study suggested that AGBE can arrest SW480 cells in S and G2/M phases.
The cytograms of annexin V analysis of SW480 cells after treatment with AGBE are shown in Fig. 6B. For the control group, the percentage of apoptotic cells was 6.5%. After treatment with 0.5 mg/mL of AGBE for 48 hours, the percentage of apoptotic cells was increased to 24.6%. Moreover, treatment with 1 mg/mL of AGBE increased the percentage of apoptotic cells to 40.9%. Data showed that AGBE can induce SW480 cell apoptosis significantly. Our flow cytometric assay results suggest that the antiproliferative effect of AGBE could be mediated by the cell cycle arrest and the induction of apoptosis.
In the developed world, human colorectal cancer is a very serious, common disease and a significant public health problem [18,19]. It has been pointed out that colorectal cancer is the third leading cause of cancer death in the USA [19]. Epidemiological studies have shown that up to 80% of colorectal cancer is attributable to diet [2,20]. Also, a diet that contains adequate or high levels of fruits and vegetables reduces risk of colon cancer [21]. Therefore, to prevent and treat this disease, it is necessary to look for and identify effective chemopreventive agents, especially from botanical resources [5].
The majority of investigations indicated that Asian ginseng root possesses anticancer activities [11,12]. As another plant in the same genus Panax, American ginseng root also possesses anticancer activities [22]. Recently, the content of ginsenosides in dried American ginseng berry was determined by HPLC, and the triterpenoid glycoside composition was compared to American ginseng root and leaf [16]. It has been observed that American ginseng berry has a distinct ginsenoside profile compared to the root and leaf. Total ginsenoside contents are different in berry, root, and leaf. The rank in order of the total ginsenoside concentration is leaf >berry>root [16,23]. The content of ginsenoside Rb3 in the berry is much higher than in the root. However, the triterpenoid glycoside content in fresh AGBE was not reported. In this study, we determined the representative ginsenoside contents in AGBE.
The purpose of the present study was to evidence the antiproliferative effect by which AGBE and ginsenosides Rb3, Re, and Rg3 inhibited SW480 colorectal cancer cell growth. Our cell proliferative assay test showed that AGBE (0.1-1.0 mg/mL) and ginsenosides Rb3, Re, and Rg3 (0.03-0.3 mg/mL) inhibited the growth of SW480 cancer cell significantly in concentration-dependent and/or time-dependent manners.
Generally, cell death may be divided into two morphologically and biochemically distinct modes, those of apoptosis and necrosis [11]. Apoptosis is characterized by cell shrinkage, nuclear and DNA fragmentation, and breaking up of the cell into membrane-bounded vesicles, termed apoptotic bodies. In our morphological observation, shrinking cells and apoptotic bodies were observed (Fig. 3B). Our 3H-thymidine assay also showed that AGBE and ginsenosides, Re, Rb3, and Rg3 suppressed cellular incorporation and may have inhibited transferring and duplication of SW480 cell's DNA.
Although the mechanism by which AGBE and ginsenosides, Re, Rb3, and Rg3 exert their inhibitive activity on the SW480 cancer cells is largely unknown, previous reports suggested that at least five possible mechanisms (antioxidant effect; activation of carcinogen metabolism; detoxification of carcinogen metabolism; anti-hormone activity; and anti-inflammatory activity) may be involved in phytochemicals providing cancer protection [11]. In this study, we carried out cell cycle and apoptotic analyses. We found that the cell growth inhibitory activities of AGBE are accomplished through cell cycle arrest and apoptosis induction. Based on our preliminary results, the inhibitory effects of the AGBE and ginsenosides Re, Rb3, and Rg3 are not due to a direct killing of the SW480 cancer cells, rather, it may likely be through effects on the cell cycle and apoptotic changes. On the other hand, antioxidant effects of AGBE and ginsenoside Re have also been identified in our previous reports [24,25]. This may play a partial role in the antiproliferation function for AGBE and its ginsenosides on colorectal cancer cells.
Data from our study showed that both AGBE and ginsenosides Rb3, Re, and Rg3 possessed significant antiproliferative effects and induced the changes of morphological appearance on SW480 human colorectal cancer cells. The mechanisms of the antiproliferation of AGBE and ginsenosides involved could be cell cycle arrest and induction of apoptosis. Further work is needed to identify responsible cell cycle and apoptotic pathways that AGBE mediated cancer cell growth inhibition.
Acknowledgments
This work was supported in part by the NIH/NCCAM grants AT003441, AT004418 and AT005362.
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249. PMID: 19474385.

2. Bingham SA. Diet and colorectal cancer prevention. Biochem Soc Trans. 2000; 28:12–16. PMID: 10816091.

3. Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995; 122:321–326. PMID: 7847642.

4. O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997; 15:246–250. PMID: 8996149.
5. Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, et al. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol. 2007; 29:175–180. PMID: 18004240.
6. Moon PD, Koo HN, Jeong HJ, Na HJ, Kim SJ, Hwang GS, et al. Haeamtang induces apoptosis of colon cancer HT-29 cells through activation of caspase-3. Am J Chin Med. 2007; 35:897–909. PMID: 17963328.

7. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58:1685–1693. PMID: 10571242.
8. Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001; 286:208–216. PMID: 11448284.

9. Xie JT, Mchendale S, Yuan CS. Ginseng and diabetes. Am J Chin Med. 2005; 33:397–404. PMID: 16047557.

10. Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007; 35:543–558. PMID: 17708622.

11. Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004; 9:259–274. PMID: 15387718.
12. Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, et al. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007; 73:669–674. PMID: 17538869.

13. Kitts D, Hu C. Efficacy and safety of ginseng. Public Health Nutr. 2000; 3:473–485. PMID: 11276295.

14. Radad K, Gille G, Liu L, Rausch WD. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006; 100:175–186. PMID: 16518078.

15. Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, et al. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006; 54:9936–9942. PMID: 17177524.

16. Wang CZ, Wu JA, McEntee E, Yuan CS. Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J Agric Food Chem. 2006; 54:2261–2266. PMID: 16536605.

17. Xie JT, Wang CZ, Ni M, Wu JA, Mehendale SR, Aung HH, et al. American ginseng berry juice intake reduces blood glucose and body weight in ob/ob mice. J Food Sci. 2007; 72:S590–S594. PMID: 17995625.

19. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96. PMID: 18287387.

20. Ryan-Harshman M, Aldoori W. Diet and colorectal cancer: review of the evidence. Can Fam Physician. 2007; 53:1913–1920. PMID: 18000268.
21. Thomson CA, LeWinn K, Newton TR, Alberts DS, Martinez ME. Nutrition and diet in the development of gastrointestinal cancer. Curr Oncol Rep. 2003; 5:192–202. PMID: 12667416.

22. Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008; 36:1019–1028. PMID: 19051332.

23. Xie JT, Mehendale SR, Wang A, Han AH, Wu JA, Osinski J, et al. American ginseng leaf: ginsenoside analysis and hypoglycemic activity. Pharmacol Res. 2004; 49:113–117. PMID: 14643691.

24. Xie JT, Shao ZH, Vanden Hoek TL, Chang WT, Li J, Mehendale S, et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006; 532:201–207. PMID: 16497296.

25. Mehendale SR, Wang CZ, Shao ZH, Li CQ, Xie JT, Aung HH, et al. Chronic pretreatment with American ginseng berry and its polyphenolic constituents attenuate oxidant stress in cardiomyocytes. Eur J Pharmacol. 2006; 553:209–214. PMID: 17092497.

Fig. 1
High performance liquid chromatographic analyses of American ginseng berry extract (AGBE). (A) Chromatogram of saponin standards, ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3, Rd, and Rg3. (B) Chromatogram of AGBE.
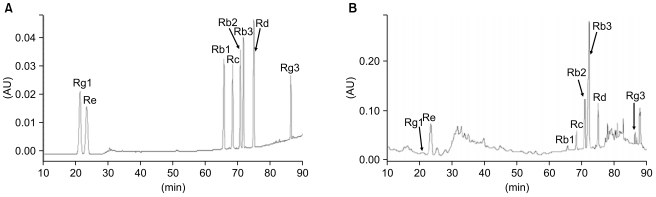
Fig. 2
Effects of American ginseng berry extract (AGBE) on proliferation of SW480 cells after treatment for different time. SW480 cells were exposed to 1.0 mg/mL of AGBE for 24, 48, 72, and 96 hr. Control at corresponding time is normalized to 100%. *p<0.05, **p<0.01 vs. corresponding control.
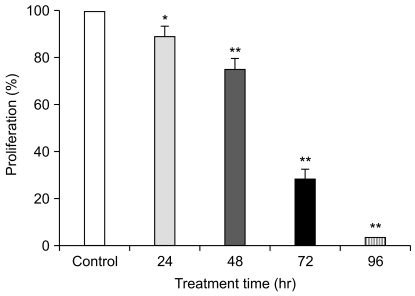
Fig. 3
Effects of American ginseng berry extract (AGBE) on proliferation and morphological changes of SW480 cells after treatment for 72 hr. (A) SW480 cells were exposed to 0.1, 0.5 and 1.0 mg/mL of AGBE for 72 hr. Control at 72 hr is normalized to 100%. *p<0.05, **p<0.01 vs. control. (B) The morphological aspects of untreated and 1 mg/mL of AGBE treated cells were photographed with a microscope.
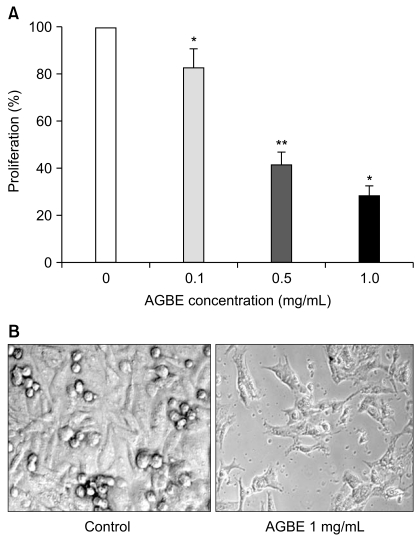
Fig. 4
Effects of ginsenosides Rb3, Re, and Rg3 on proliferation of SW480 cells. Cells were treated with 0.03, 0.1 and 0.3 mg/mL of ginsenosides for 72 hr. Control at 72 hr is normalized to 100%.
Fig. 5
Effects of American ginseng berry extract (AGBE) and ginsenosides on the 3H-thymiding incorporation of SW480 cells. (A) SW480 cells were treated with 0.5 and 1.0 mg/mL of AGBE for 72 hr. (B) SW480 cells were treated with 0.03, 0.1, and 0.3 mg/mL of ginsenosides Rb3, Re, and Rg3 for 72 hr. Control at 72 hr is normalized to 100%. **p<0.01 vs. control.
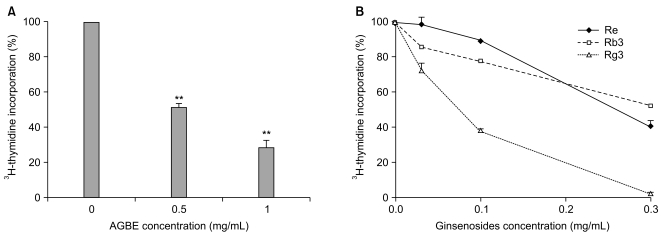
Fig. 6
Cell cycle and apoptosis analysis of SW480 cells using flow cytometry. SW480 cells were treated with 0.5 and 1.0 mg/mL of American ginseng berry extract (AGBE) for 48 hr. (A) Cell cycle profile of SW480 cells. Since not enough viable cells were collected after treatment with 1 mg/mL of AGBE, the cell cycle profile of this group was not calculated. (B) The cytograms of apoptosis assay of SW480 cells. The percentage of apoptotic cells was indicated.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download