Abstract
Purpose
This phase II clinical trial was conducted to evaluate the activity and safety of a combination treatment of paclitaxel (Genexol®) plus carboplatin in patients with advanced non-small cell lung cancer.
Materials and Methods
Chemotherapy-naïve patients having histologically confirmed advanced or metastatic non-small cell lung cancer were enrolled. Genexol® was administered at 225 mg/m2 intravenous (IV) infusion over 3 hours, followed by carboplatin (area under the concentration-time curve=6) IV on day 1 every 3 weeks.
Results
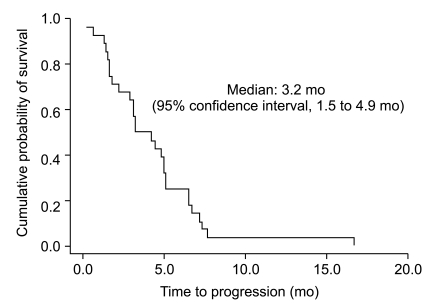
Twenty-eight patients were enrolled between January 2003 and January 2005. A total of 110 cycles of chemotherapy were given. The median number of chemotherapy cycles was 4. A total of 25 study patients were evaluable. On an intent-to-treat basis, there were ten partial responses (response rate 35.7%). The median time-to-progression was 3.2 months (95% confidence interval [CI], 1.5 to 4.9) and the median overall survival was 8.2 months (95% CI, 4.1 to 12.3). The main hematologic grade 3/4 toxicity was neutropenia, which was observed in 14 (50.0%) patients. The main non-hematologic toxicity was peripheral neuropathy, which was observed in 12 patients (42.9%). Grade 3/4 neuropathy occurred in 8 patients (28.6%) and three patients discontinued treatment because of neuropathy.
Lung cancer is the fourth most common cancer and the leading cause of cancer deaths in Korea [1].The platinum-based regimen using paclitaxel, gemcitabine and docetaxel has shown high response rates and a survival benefit for patients with advanced non-small cell lung cancer (NSCLC). No significant difference in survival was observed among four commonly used regimens, but the combination regimen of paclitaxel and carboplatin had a lower rate of toxic effects than the other regimens [2,3]. When considering toxicity profile, a combination chemotherapy of paclitaxel and carboplatin is an appropriate option in advanced or metastatic NSCLC. Previously, paclitaxel (Taxol®, Bristol-Myers Squibb Co., Princeton, NJ) was obtained using a semisynthetic process from Taxus baccata, but another paclitaxel, Genexol® (Samyang Genex Co., Seoul, Korea) was produced from Taxus chinensis using plant cell culture technology. This technology enables the manufacture of Genexol® with a high purity, low purification cost, constant yield, and without environmental problems. In a phase II trial with advanced NSCLC patients, combination chemotherapy using Genexol® and cisplatin showed no less response than a previous study and had no significant toxicity [4]. We conducted a phase II trial to evaluate the safety and efficacy of a combination treatment of another paclitaxel (Genexol®) plus carboplatin in patients with advanced NSCLC.
The eligibility criteria were as follows: 1) histologically-confirmed inoperable, locally advanced or metastatic NSCLC, 2) patients between 18 and 75 years of age, 3) no previous chemotherapy, 4) an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, 5) no serious or uncontrolled concurrent medical illness, 6) at least one radiographically measurable lesion, and 7) adequate baseline hematologic function, defined as (an absolute neutrophil count [ANC] ≥1.5×109/L, and a platelet count ≥100×109/L), adequate hepatic function (serum bilirubin ≤1.25×upper normal limit [UNL], serum aspartate aminotransferase alanine aminotransferase ≤2.5×UNL and serum alkaline phosphatase ≤5.0×UNL), and adequate renal function (serum creatinine ≤1.5 mg/dL). 8) All patients gave their written informed consent to participate in the study, which was approved by the local institutional review boards. The exclusion criteria included the following: 1) pre-existing peripheral neuropathy, 2) concurrent or prior malignancy, except curatively resected cervical carcinoma in situ or squamous cell carcinoma of the skin, 3) active infection, 4) brain metastasis, 5) pregnancy confirmed, and 6) concurrent treatments that interfered with the study's evaluation.
Genexol® was supplied by Samyang Genex Co. as a concentrated sterile solution for intravenous (IV) administration in a 5 mL vial containing 30 mg of paclitaxel in polyoxyethylated castor oil and dehydrated alcohol (Cremophor EL/ethanol). The patients received Genexol®, 225 mg/m2 as a 3-hour IV infusion followed by carboplatin, area under the concentration-time curve (AUC)=6, as a 1-hour IV infusion on day 1 every 3 weeks. The Genexol® was given with premedication as follows: dexamethasone 10 mg (or its equivalent) IV administered 12 hours and 30 minutes before the Genexol®, chlorpheniramine maleate 4 mg (or its equivalent) IV, ranitidine 50 mg (or its equivalent) IV, 30 minutes before the Genexol®. Antiemetic prophylaxis was given according to a local protocol. In the absence of progressive disease or intolerable toxicity, the patients were treated for a maximum of six cycles. Patients who achieved a complete or partial response (PR) could receive two additional cycles of chemotherapy after best response, to a maximum 8 cycles. Granulocyte colony-stimulating factor was not routinely used in the study.
Each cycle was started if the ANC and platelet counts on the day of treatment were ≥1.5×109/L and ≥100×109/L, respectively. In the case of any grade 3 or greater toxicity, except alopecia, chemotherapy was delayed 1 week, then restarted after recovery. The patients were treated at three dose levels (0, -1, -2) as follows: enrolled patients were started at dose level 0, in which they received Genexol®, 225 mg/m2, plus carboplatin, AUC=6. If grade 3/4 thrombocytopenia or febrile neutropenia, grade 4 neutropenia, or grade 3/4 non-hematologic toxicity were observed in the previous cycle, the dose of test drugs was decreased to level -1 (Genexol®, 180 mg/m2, plus carboplatin, AUC=5). If the above toxicities occurred after dose reduction, the dose of drugs was decreased to level -2 (Genexol®, 135 mg/m2, plus carboplatin, AUC=4). If a recovery was still not achieved by day 35 of treatment, the patient was withdrawn from the study.
A pretreatment evaluation was performed within 2 weeks of the therapy initiation, including full history, physical examination, complete blood cell counts (CBC) with the differential count, serum biochemistry, chest X-ray, chest computed tomography (CT), Brain CT and site-specific imaging. The response to treatment was assessed according to the response evaluation criteria in solid tumors (RECIST) criteria. All the patients who received at least one cycle of chemotherapy were considered evaluable for toxicity. CT scans of the measurable lesions were performed within 2 weeks prior to treatment. After every 2 cycles of chemotherapy, CT scans were performed to determine the response of the disease, or sooner if there was evidence of any clinical deterioration. CBC with the differential count and serum biochemistry were repeated just before the start of each chemotherapy cycle, and the toxicity was graded according to the National Cancer Institute common toxicity criteria (NCI-CTC version 2.0).
The trial was conducted according to the group sequential design with the response rate as the primary efficacy endpoint. The number of patients required for the study was calculated based on a targeted response rate of 45% and a minimum response rate of 25%, with α error of 0.1 and β error of 0.2. Analysis of the clinical efficacy was performed according to an intent-to-treat analysis. All patients who received at least two cycles of treatment, or progressed after one course of chemotherapy, were assessable for response and patients who received at least one course were assessable for toxicity. The time to progression (TTP) was calculated from the 1st day of chemotherapy to the date of disease progression or the last examination, and the overall survival (OS) from the first day of chemotherapy to the date of death or the last follow-up. The Kaplan-Meier method was used to analyze the TTP and OS, and the 95% confidence interval (CI) for the median time to event was computed. The actual delivered dose-intensity (DI) was calculated as the ratio of the total dose (expressed in mg) per m2 actually received by the patient, divided by the actual total treatment duration expressed in weeks. The relative DI was calculated as the ratio of the actual delivered DI to the DI planned by the protocol. The SPSS ver. 14.0 (SPSS Inc., Chicago, IL) were used for the statistical computation.
Between January 2003 and January 2005, 28 patients with a median age of 63 years (range, 45 to 73 years) from Seoul, Bucheon, and Cheonan Soonchunhyang University Hospital were studied. The patient baseline characteristics are listed in Table 1. There were 20 men and 8 women, and 10 patients (35.7%) had an ECOG performance status of 2. Twenty patients (71.4%) had primary metastatic disease and 8 patients (28.6%) had stage IIIb disease. The most common histologic type of tumor was adenocarcinoma.
Twenty-five of the enrolled 28 patients were evaluable for response according to the RECIST criteria. Three patients were excluded from the response analysis because these patients declined continuation of treatment for voluntary withdrawal after one cycle. Ten patients (35.7%) achieved a PR, 7 patients (25.0%) had stable disease, and 8 patients (28.6%) progressed during the course of treatment. None of the patients achieved a complete response. From intent-to-treat analysis, the overall response rate was 35.7%. The median follow-up duration was 8.1 months (range, 0.4 to 23.5 months). All patients were dead. The TTP and OS were 3.2 months (95% CI, 1.5 to 4.9 months) (Fig. 1) and 8.2 months (95% CI, 4.1 to 12.3 months) (Fig. 2), respectively.
One hundred ten chemotherapy cycles were administered to the 28 patients. The median number of cycles was 4 (range, 1 to 8). Table 2 summarizes the incidence of the main toxicities. The major grade 3-4 hematologic toxicity was neutropenia (50.0%). However, febrile neutropenia was not encountered. The main non-hematologic toxicity was peripheral neuropathy, which was observed in 12 patients (42.9%). Grade 3/4 neuropathy occurred in 8 patients (28.6%), and three patients (10.7%) discontinued the treatment because of peripheral neuropathy. A dose reduction was required in 12 patients; eight patients, due to peripheral neuropathy, and four patients, due to grade 4 neutropenia. A grade 3-4 hypersensitivity reaction to Genexol® was not observed. The relative dose intensities of Genexol® and carboplatin were 74.4% and 77.2% of planned doses, respectively. There were no treatment-related deaths.
Platinum-based combination chemotherapy has shown a high response rate and survival benefit for patients with advanced or metastatic NSCLC, but none of the combination chemotherapy regimens (paclitaxel+cisplatin vs. gemcitabine+cisplatin vs. docetaxel+cisplatin vs. paclitaxel+carboplatin) have shown a significant advantage over the others for the treatment of advanced NSCLC [2]. The major toxicities of paclitaxel are hypersensitivity, myalgia and peripheral neuropathy. The use of carboplatin instead of cisplatin may reduce the occurrence of peripheral neurotoxicity, and by using the AUC, myelotoxicity can be manageable [5]. Based on this evidence, we investigated the activity and safety of a combination treatment of Genexol® plus carboplatin in patients with advanced NSCLC. Combination chemotherapy with Genexol® plus cisplatin showed no less response than the previous study, with no considerable toxicity [4]. However, the result of a Genexol® plus carboplatin combination regimen for treating NSCLC was not published.
The results of this study showed that the regimen is an effective combination chemotherapy as first-line treatment for advanced NSCLC. The response rate, TTP and OS of this trial were 35.7%, 3.2 months and 8.2 months, respectively. These results were within the range of previous trials, where platinum-based chemotherapy was used for treatment of advanced NSCLC [2,3,6,7]. A severe hypersensitivity reaction caused by paclitaxel has been reported to develop in 1 to 3% of patients during a 3-hour infusion, however, we observed no patient who experienced a grade 3/4 of hypersensitivity reaction in this trial [2,8]. Myelotoxicity was not greater than in the previous report and no toxic death was noticed. Neutropenia was the most frequent hematologic toxicity, although no febrile neutropenia was seen. Except for neurotoxicity, non-hematologic problems were mild and manageable.
Neurotoxicity was the most serious problem. It was observed in 12 patients (42.9%), and grade 3/4 neuropathy occurred in 8 patients (28.6%). Three patients (10.7%) discontinued the treatment because of peripheral neuropathy, however, all these patients achieved a partial response. A dose reduction was required in 8 patients due to peripheral neuropathy, however, based on univariate analysis, grade 3/4 neuropathy was not a significant factor affecting OS and response. Of the 8 patients who required dose reduction, 5 patients (62.5%) had an ECOG performance status of 2.
Neurotoxicity was more common in patients with poor performance status in our study. Although we used carboplatin, instead of cisplatin to reduce neurotoxicity, the rate of grade 3/4 neuropathy was relatively high compared to other studies [2]. Perhaps the rate of toxicity reflected the relatively high dose of Genexol®. A higher dose of paclitaxel (225 mg/m2 vs. 175 mg/m2) combined with carboplatin (AUC=6) prolongs the median time to progression but causes more neurotoxicity and neutropenia [8-10]. However, to make such an assumption, more evidence should be provided, i.e., 175 mg/m2 vs. 225 mg/m2 of Genexol® in caucasian and asian patients.
Although our study was not a randomized trial comparing paclitaxel plus carboplatin combination chemotherapy, Genexol® and carboplatin is an effective regimen for patients with advanced or metastatic NSCLC, and our results indicate that, when used with this schedule, Genexol® dose not appear to be safe from a neurotoxicity. Modest dose reduction of Genexol® is needed.
Acknowledgments
We would like to thank Samyang Genex Corporation, Korea for donating the study drugs.
References
1. Ministry for Health, Welfare and Family Affairs, Headquarters of Central Cancer Registry. 2007 Annual report of the Korea Cancer Registration. 2009. Seoul: Ministry for Health, Welfare and Family Affairs.
2. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98. PMID: 11784875.

3. Kelly K, Crowley J, Bunn PA Jr, Presant CA, Grevstad PK, Moinpour CM, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001; 19:3210–3218. PMID: 11432888.

4. Lee SH, Park K, Suh C, Kim HK, Kim JS, Im YH, et al. A multi-center, phase II clinical trial of Genexol® (paclitaxel) and cisplatin for patients with non-small cell lung cancer. Cancer Res Treat. 2003; 35:30–34.
5. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655. PMID: 7165009.

6. Smit EF, van Meerbeeck JP, Lianes P, Debruyne C, Legrand C, Schramel F, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer. A phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group: EORTC 08975. J Clin Oncol. 2003; 21:3909–3917. PMID: 14581415.
7. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–3551. PMID: 18506025.

8. Kosmidis P, Mylonakis N, Skarlos D, Samantas E, Dimopoulos M, Papadimitriou C, et al. Paclitaxel (175 mg/m2) plus carboplatin (6 AUC) versus paclitaxel (225 mg/m2) plus carboplatin (6 AUC) in advanced non-small-cell lung cancer (NSCLC): a multicenter randomized trial. Hellenic Cooperative Oncology Group (HeCOG). Ann Oncol. 2000; 11:799–805. PMID: 10997806.
9. Morère JF, Piperno-Neumann S, Coulon MA, Vaylet F, L'Her P, Brunet A, et al. Dose-finding study of paclitaxel and carboplatin in patients with advanced non-small cell lung cancer. Anticancer Drugs. 2000; 11:541–548. PMID: 11036956.

10. Huizing MT, Giaccone G, van Warmerdam LJ, Rosing H, Bakker PJ, Vermorken JB, et al. Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dosesequencing study in patients with non-small-cell lung cancer: the European Cancer Centre. J Clin Oncol. 1997; 15:317–329. PMID: 8996159.

Table 1
Patient characteristics
Table 2
Toxicities according to National Cancer Institute common toxicity criteria




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download