Abstract
Glioblastoma multiforme (GM) is one of the most aggressive primary brain tumors, and has a poor prognosis despite intensive treatment. GM is also the most malignant astrocytoma, with histopathological features that include cellular polymorphism, rapid mitotic activity, microvascular proliferation, and necrosis. The causes of GM remain obscure, but several reports have shown associations between GM and genetic alterations and radiation exposure. Furthermore, high-dose chemotherapy/radiotherapy with autologous stem cell transplantation is increasingly being used to treat patients with leukemia, and patients who undergo stem cell transplantation have a higher risk of solid tumor cancer development later in life. Based on these associations, we discuss GM development in a patient who underwent chemoradiotherapy conditioning prior to stem cell transplantation.
Glioblastoma multiforme (GM) is the most common type of primary brain tumor. It is found in all age groups, but is more frequently encountered in the elderly. The etiology of GM is not clear, but it is known to be associated with radiation therapy [1] and diverse genetic defects [2,3]. Surgery, followed by standard radiotherapy with concomitant chemotherapy, is the standard of care [4]. However, despite aggressive treatment, the prognosis remains poor, with a median survival of 12-15 months. This dismal prognosis is associated with genetic abnormalities, which result in the aberrant activation or suppression of cellular signal transduction pathways and subsequent resistance to radiation and chemotherapy.
GM has been previously reported in patients with hematologic disorders who have received stem cell transplantation (SCT) after being conditioned with chemotherapy and radiation [5], but no such case has been reported in Korea. We encountered one 39-year-old patient with acute myelogenous leukemia (AML), who developed GM 61 months after autologous hematopoietic stem cell transplantation (ASCT). We present this case and include a review of the literature.
A 39-year-old male was admitted to our hospital with loss of memory for 10 months. The patient was diagnosed with AML in February 2001 and underwent ASCT in October 2001. No specific complications had been detected after transplantation until the patient visited our hospital with a chief complaint of continued loss of memory since the end of 2005. The patient underwent a brain computed tomography scan and surgery was planned based on a suspicion of GM. However, the patient suffered rapid memory loss 3 days before hospitalization. Exacerbation of brain edema was suspected and he was admitted to the emergency room.
The patient underwent induction therapy consisting of idarubicin (IDA, 12 mg/m2/day), N4-behenoyl-1-β-D-arabinofuranosylcytosine (BH-AC, 300 mg/m2/day), and augmentation (BH-AC, 400 mg/m2/day). In May 2001, he received the first consolidation therapy (IDA, 12 mg/m2/day; BH-AC, 300 mg/m2/day), and in July he underwent a second consolidation therapy (mitoxantrone, 12 mg/m2/day; etoposide phosphate, 100 mg/m2/day). In October of the same year, he received ASCT after being conditioned with cytarabine (Ara-C, 1.5 g/m2/day) for 3 days, and receiving total body irradiation (TBI, 10 Gy for 3 days), and melphalan (100 mg/m2/day) for 1 day. He was subsequently monitored, but no transplantation-related symptoms were noted. Memory loss, however, developed at the end of 2005, which prompted his visit to our Internal Medicine Department.
Vital signs at patient presentation were: blood pressure, 135/80 mm Hg; pulse, 93/min; respiration rate, 20/min; and body temperature, 37.1℃. There was no sign of acute illness. A head and neck examination showed no signs of pale conjunctiva, pharyngeal injection, or lymph node enlargement. A chest examination showed normal lung and heart sounds, an abdominal examination showed no signs of an enlarged liver or spleen, tenderness, or rebound tenderness, and a neurological examination showed that cerebral nerves, motor nerves, and sensory nerves were all normal.
A complete blood count showed a total white blood cell count of 15.2×109/L (neutrophils, 84.6%; lymphocytes, 13.5%; and monocytes, 1.8%), hemoglobin 14.5 g/dL, and platelets 195×109/L. All additional blood chemistry results were within the normal range.
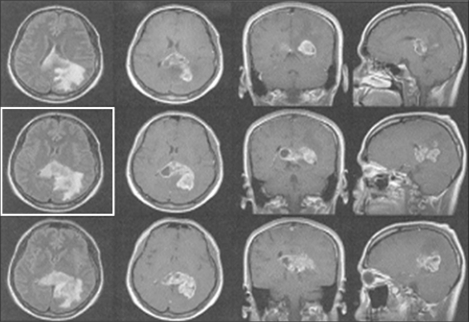
Brain magnetic resonance imaging (MRI) conducted before his visit to the hospital revealed irregular, leaf-like lesions (5.5×3.6×3.2 cm) in the parietal and occipital lobes, over medial temporal lobes and the splenium of the corpus callosum. The lesion contained internal cysts and was associated with edema. MRI also revealed meningeal metastasis (Fig. 1).
Pathologic findings of malignant astrocytes confirmed GM grade 4 according to World Health Organization (WHO) criteria. Additional microscopic analysis showed prominent cellularity, moderate cytological pleomorphism, 5-6 mitoses per 10 high-power fields, and endothelial cell proliferation and necrosis. An immunohistochemical examination confirmed a positive reaction for glial fibrillary acidic protein, the intermediate filament specifically generated in central nervous system (CNS) astrocytoma. Furthermore, 75% of cells were positive for Ki-67 protein, nuclear and nucleolar proteins, which are found within nuclei only during the interphase stage of cell division, and are associated with cell proliferation. Positive reactions were also observed for O6-methylguanine-DNA methyltransferase (MGMT), and for epidermal growth factor receptor (EGFR).
On the 5th day of hospitalization, frameless neuronavigation guided craniotomy was performed with open biopsy for pathologic confirmation, and concomitant chemoradiotherapy (180 cGy/day radiation plus temozolomide 75 mg/m2/day) was maintained from day 11 until day 19. Following 2 months of hospitalization, the patient was discharged without completing a second maintenance therapy because he refused further chemoradiotherapy. Four months after diagnosis of GM, he reported sudden dyspnea and was treated in the intensive care unit. He succumbed to acute respiratory distress syndrome, which developed from Pneumocystis carinii pneumonia, despite the administration of trimethoprime/sulfamethoxazole for 11 days.
The use of SCT to treat hematologic disorders is increasing, and transplantation-conditioning regimens usually incorporate chemotherapy and TBI. However, these two treatment modalities are carcinogenic and their usages have generated increasing interest in their association with secondary tumor development after transplantation. Furthermore, several studies have been conducted on the incidence of secondary tumor development and the risk factors involved [6,7]. The incidence of solid tumors in patients who received SCT (including autologous and allogeneic) has been reported to be 8.3-fold greater than in the normal population at 10 year post-SCT, and incidences in the brain and other parts of the CNS were found to be 7.6-fold greater. Secondary solid tumors that form after ASCT are likely to be directly influenced by high-dose chemotherapy and TBI, and the development of GM after SCT is no exception [8]. Alkylating anti-cancer agents are known to be capable of generating secondary tumors after being used for treatment of a primary cancer. The alkylating agent melphalan is frequently used in conditioning regimens, and the incidence of secondary tumor development after treatment with melphalan is 7-fold greater than normal, and is 10-fold greater after melphalan/TBI conditioning. This finding concurs with a previous report that the most significant risk factor for the development of a secondary tumor is the combined use of radiotherapy and chemotherapy [9]. Furthermore, this risk is much higher for cancers of the skin, brain, breast, or thyroid gland [10].
According to the assessment based on the Naranjo Causality Scale [11], which addresses the strength of the relationship between a drug and a suspected adverse reaction, our case had a total score of 6, based on the following scale: (definite causal relationship when the total score is ≥9, probable relationship when between 5-8, possible relationship when between 1-4, and doubtful relationship for a total score of 0). The score of 6 was calculated based on responses to the following four questions: (Are there previous conclusive reports on reaction? Yes=+1; Did the adverse event appear after the suspected drug was administered? Yes=+2; Are there alternative causes that could, on their own, have caused the reaction? No=+2; Was the reaction more severe when the dose was increased, or less severe when the dose was decreased? Yes=+1).
According to this scale, in our case, an association between melphalan treatment and the incidence of GM was likely. Additionally, in a previous study, based on multivariable analysis of TBI as a potential factor for secondary tumor generation, the incidence of a secondary tumor among patients with a hematopoietic disorder who received TBI was 3.9-fold greater than that of patients who did not receive TBI [12]. In the described case, 10 Gy of radiation was administered over 3 days, which would have increased the risk of GM development based on the above mentioned analysis [13]. Accordingly, in our patient, chemotherapy or TBI after SCT may have generated the secondary solid tumor and their simultaneous application would have increased the risk of a second malignancy.
Secondary glioblastoma is distinct from primary glioblastoma, and develops as a result of different genetic alterations [14]. Because the patient demonstrated EGFR amplification, which is a genetic alternation typical of primary GM, the proper diagnosis was "second primary GM developing after chemoradiotherapy," rather than secondary GM. Despite their distinctive pathogeneses, their clinical courses and treatments are similar to those of primary disease in terms of widespread invasion and resistance to therapy. Important prognostic factors include the type of surgery and adjuvant chemotherapy [15]. Several studies reported that at least 4 cycles of radiotherapy plus adjuvant chemotherapy (especially, temozolomide) are required to obtain optimal responses. Based on the possibility of generating a secondary tumor, regular examinations are required to detect minute physical changes in patients who undergo SCT.
Second primary GM occurring in a 39-year-old male patient with AML who had undergone ASCT may have been caused by melphalan or TBI. A similar case has not been previously reported in Korea, and thus, we present this case and include a literature review.
References
1. Kitanaka C, Shitara N, Nakagomi T, Nakamura H, Genka S, Nakagawa K, et al. Postradiation astrocytoma: report of two cases. J Neurosurg. 1989; 70:469–474. PMID: 2536806.
2. Nagarajan RP, Costello JF. Epigenetic mechanisms in glioblastoma multiforme. Semin Cancer Biol. 2009; 19:188–197. PMID: 19429483.

3. Yadav AK, Renfrow JJ, Scholtens DM, Xie H, Duran GE, Bredel C, et al. Monosomy of chromosome 10 associated with dysregulation of epidermal growth factor signaling in glioblastomas. JAMA. 2009; 302:276–289. PMID: 19602687.

4. Minniti G, Muni R, Lanzetta G, Marchetti P, Enrici RM. Chemotherapy for glioblastoma: current treatment and future perspectives for cytotoxic and targeted agents. Anticancer Res. 2009; 29:5171–5184. PMID: 20044633.
5. Deeg HJ, Sanders J, Martin P, Fefer A, Neiman P, Singer J, et al. Secondary malignancies after marrow transplantation. Exp Hematol. 1984; 12:660–666. PMID: 6386505.
6. Forrest DL, Nevill TJ, Naiman SC, Le A, Brockington DA, Barnett MJ, et al. Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant. 2003; 32:915–923. PMID: 14561993.

7. Ghelani D, Saliba R, Lima M. Secondary malignancies after hematopoietic stem cell transplantation. Crit Rev Oncol Hematol. 2005; 56:115–126. PMID: 15979325.

8. Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socíe G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997; 336:897–904. PMID: 9070469.

9. Mauch PM, Kalish LA, Marcus KC, Coleman CN, Shulman LN, Krill E, et al. Second malignancies after treatment for laparotomy staged IA-IIIB Hodgkin's disease: long-term analysis of risk factors and outcome. Blood. 1996; 87:3625–3632. PMID: 8611686.

10. Kulkarni S, Powles R, Treleaven J, Singhal S, Horton C, Sirohi B, et al. Melphalan/TBI is not more carcinogeneic than cyclophosphamide/TBI for transplant conditioning: follow-up of 725 patients from a single centre over a period of 26 years. Bone Marrow Transplant. 2000; 25:365–370. PMID: 10723578.
11. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30:239–245. PMID: 7249508.

12. Leiper AD. Late effects of total body irradiation. Arch Dis Child. 1995; 72:382–385. PMID: 7618901.

13. Salvati M, D'Elia A, Melone GA, Brogna C, Frati A, Raco A, et al. Radio-induced gliomas: 20-year experience and critical review of the pathology. J Neurooncol. 2008; 89:169–177. PMID: 18566750.

14. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007; 170:1445–1453. PMID: 17456751.

15. Erpolat OP, Akmansu M, Goksel F, Bora H, Yaman E, Büyükberber S. Outcome of newly diagnosed glioblastoma patients treated by radiotherapy plus concomitant and adjuvant temozolomide: a long-term analysis. Tumori. 2009; 95:191–197. PMID: 19579865.

Fig. 1
Brain magnetic resonance imaging. An irregular and nodular enhancing lobulated lesion of -5.5×3.6×3.2 cm size is noted in the parieto-occipital region extending splenium of corpus callosum and medial temporal lobe. This lesion has an internal cystic portion and is associated with surrounding edema. Leptomeningeal enhancement is noted in both the lateral, 4th ventricle and perimesencephalic cystern, suggesting leptomeningeal seeding. There is no evidence of focal stenotic lesion or aneurismal dilatation.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download