Abstract
Painful ophthalmoplegia (PO) and concomitant numb chin syndrome (NCS) is a very rare event. There are a few reports in the literature about PO and concomitant NCS that have preceded the diagnosis of a malignancy. In this report, we describe a patient with diffuse large B cell lymphoma who presented with PO and concomitant NCS as the initial symptom of the disease.
Painful ophthalmoplegia (PO) refers to periorbital pain plus ipsilateral ocular motor nerve palsy with or without Horner's syndrome, whereas numb chin syndrome (NCS) indicates facial and oral numbness within the mental nerve distribution. These two clinical entities may be the first signs of a systemic malignancy, even in the absence of other cancer-associated symptoms [1-8].
A 71-year-old man was admitted to our neurology department because of double vision and numbness on both sides of the chin. Six days before admission, the patient awoke with severe pain radiating from the left periorbital area to the temporal region. The pain was precipitated by eye movements. Within a day, he developed binocular diplopia and soon noticed numbness on both sides of his chin. The patient had no ongoing health problems except for a 4-year history of well-controlled hypertension. A review of organ systems was non-contributory, and there was no history of facial trauma or dental treatment.
At admission, the patient was in good condition. There was no clinical evidence of palpable regional lymph nodes or other abnormal findings on the physical exam. The neurological examination revealed a left 6th nerve palsy and hypoesthesia involving the area from the chin to the lower lip on both sides. The response to touch and pinprick was markedly decreased in the chin region (about 20% of normal sense).
Laboratory studies, including a complete blood count and blood chemistry panel showed mild leukocytosis (white blood cell count, 11,140/mm3) and increased liver enzymes (aspartate transaminase, 138 U/L; alanine transaminase, 101 U/L; lactate dehydrogenase, 2,319 U/L). An elevated erythrocyte sedimentation rate (26 mm/hr) and an elevated C-reactive protein (10.88 mg/dL) level were noted. T1-weighted contrast-enhanced magnetic resonance imaging of the brain showed a slightly enhanced mass lesion in the left cavernous sinus (Fig. 1). Cerebrospinal fluid studies, including cytology, were normal (pressure, 140 mm H2O; white blood cells, 0; glucose, 56.5 mg/dL; protein, 39 mg/dL). On the 3rd hospital day, we did positron emission tomography using 18F-fluorodeoxyglucose (FDG-PET), which disclosed intense FDG accumulation in multiple organs, including abdominal and pelvic organs (large intestines, rectum, peritoneum, omentum, mesentery, and left adrenal gland), skeletal system (maxilla, mandible, vertebrae, sternum, ribs, clavicles, scapulae, pelvic bones, humeri, and femurs), left pleura, bilateral skull base, and left cavernous sinus (Fig. 2). The maximum standardized uptake values were 12.4 in the ascending colon, 10.4 in the rectum, 10.0 in the omentum, 9.7 in the left pleura, and 7.6 in the left cavernous sinus. On the 5th hospital day, colonoscopy was done and a 5×6 cm ulceroinfiltrative mass lesion in the ascending colon near the ileocecal valve was observed. In addition, a 3.5 cm round submucosal mass lesion was seen in the rectum; the lesion was firm and fixed. The biopsy specimen obtained from the colorectum revealed a diffuse infiltrate of atypical lymphoid cells (Fig. 3A). Immunohistochemistry was strongly positive for the B-cell marker CD20 (Fig. 3B) and revealed a high proliferation index (ki-67=90%) (Fig. 3C) but was negative for T-cell marker CD3, or cytokeratin AE1/AE3. The morphologic features together with the immunohistochemical findings were diagnostic of a diffuse large B-cell lymphoma and not carcinoma. The subsequent bone marrow biopsy demonstrated a marked reduction of normal hematopoietic cells and numerous large atypical lymphoid cells, consistent with bone marrow involvement of large B cell lymphoma.
On the 8th hospital day, the patient was treated with intravenous dexamethasone prior to chemotherapy. On the 12th hospital day, he complained of acute abdominal pain and underwent an emergency abdominal computed tomography which showed a markedly thickened terminal ileal loop, cecum, and appendix with a pneumoperitoneum, suggestive of a bowel perforation. An explorative laparotomy was performed under the diagnosis of ileocecal perforation due to malignant lymphoma, and a right hemicolectomy and distal ileectomy were done. He expired on the 11th postoperative day secondary to sepsis.
The differential diagnosis of PO involves numerous diseases, including neoplasms (i.e., primary parasellar tumors, and local or distant metastases), vascular disease (i.e., aneurysm and carotid dissection), inflammatory disease (i.e., orbital pseudotumor, sarcoidosis, and Tolosa-Hunt syndrome), infectious etiologies (i.e., fungal and mycobacterial), opthalmoplegic migraine, and diabetic ophthalmoplegia [9]. PO caused by neoplasm occurs through direct compression in the orbit, superior orbital fissure, or cavernous sinus. The cavernous sinus involvement of a neoplasm results from direct invasion by parasellar lesions (e.g., pituitary adenoma, craniopharyngioma, and meningioma), perineural spread from head and neck malignancies, and hematogenous spread from distant cancers [9-11]. As in this case, symptoms from cavernous sinus metastasis may be the first sign of an underlying occult malignancy (Table 1) [1-3]. On the day of admission, the patient presented herein did not have any other constitutional symptoms, except a painful abducens nerve palsy and numbness of the chin.
NCS, or mental neuropathy, has been reported in inflammatory disorders (i.e., sarcoidosis and connective tissue diseases), trauma, and tumors, and cysts involving the mandible [5,12]. Non-traumatic NCS may be an initial sign of neoplastic infiltration of the mental canal by malignancy [1,2,4-7]. Non-traumatic NCS is usually unilateral, but may be bilateral, as in our case [2,8]. NCS may precede the diagnosis of malignancy in nearly one-half of cases [7]. In addition, in 67% of the patients with systemic malignancy, NCS may occur in association with progression of the disease, while in 31%, it may signify a relapse [8]. Thus, the clinical importance of this neuropathy is its possible link to systemic malignancy as the initial symptom, and as a sign of relapse or progression.
In our case, the patient developed a PO and concurrent NCS. The combination of the two clinical symptoms has been reported exclusively in several hematologic malignancies (Burkitt-type lymphoma/leukemia) [1,2]. With this in mind, we did an FDG-PET scan, which has proven useful in the staging of hematological malignancies, although the patient had no constitutional symptoms of a lymphoma, such as a fever, malaise, fatigue, or myalgia. Choosing this evaluation process can be argued due to a priori selection of diagnostic method. However, we think the combination of the two clinical manifestations can signify the presence of a systemic malignancy, such as a hematopoietic cancer.
PET is a new diagnostic technique that is used to differentiate benign from malignant tumors and to detect metastasis and lymph node involvement in primary cancer, but little has been published about its possible use in the detection of unknown primary tumors. Here we report the case of a man who suffered from PO and NCS as the sole symptoms of a malignant lymphoma. This report alerts neurologists and general practitioners that sometimes care for patients with similar neurological symptoms. In this particular case, FDG-PET proved to be a very useful diagnostic method for early recognition.
References
1. Seixas DV, Lobo AL, Farinha NJ, Cavadas L, Campos MM, Ayres-Basto M, et al. Burkitt leukemia with numb chin syndrome and cavernous sinus involvement. Eur J Paediatr Neurol. 2006; 10:145–147. PMID: 16621630.

2. Lau JJ, Okada CY, Trobe JD. Galloping ophthalmoplegia and numb chin in Burkitt lymphoma. J Neuroophthalmol. 2004; 24:130–134. PMID: 15179066.
3. Heo JH, Park KD, Sunwoo IN. A case of painful ophthalmoplegia associated with pelvic malignant lymphoma. J Korean Neurol Assoc. 1987; 5:272–276.
4. Kuklok KB, Burton RG, Wilhelm ML. Numb chin syndrome leading to a diagnosis of acute lymphoblastic leukemia: report of a case. J Oral Maxillofac Surg. 1997; 55:1483–1485. PMID: 9393412.

5. Hiraki A, Nakamura S, Abe K, Takenoshita Y, Horinouchi Y, Shinohara M, et al. Numb chin syndrome as an initial symptom of acute lymphocytic leukemia: report of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997; 83:555–561. PMID: 9159815.
6. Recht L, Mrugala M. Neurologic complications of hematologic neoplasms. Neurol Clin. 2003; 21:87–105. PMID: 12690646.

7. Massey EW, Moore J, Schold SC Jr. Mental neuropathy from systemic cancer. Neurology. 1981; 31:1277–1281. PMID: 6287347.

8. Lossos A, Siegal T. Numb chin syndrome in cancer patients: etiology, response to treatment, and prognostic significance. Neurology. 1992; 42:1181–1184. PMID: 1603345.

9. Gladstone JP. An approach to the patient with painful ophthalmoplegia, with a focus on Tolosa-Hunt syndrome. Curr Pain Headache Rep. 2007; 11:317–325. PMID: 17686398.

10. Keane JR. Cavernous sinus syndrome: analysis of 151 cases. Arch Neurol. 1996; 53:967–971. PMID: 8859057.

11. Lanzino G, Hirsch WL, Pomonis S, Sen CN, Sekhar LN. Cavernous sinus tumors: neuroradiologic and neurosurgical considerations on 150 operated cases. J Neurosurg Sci. 1992; 36:183–196. PMID: 1306200.
12. Horowitz SH. Isolated facial numbness: clinical significance and relation to trigeminal neuropathy. Ann Intern Med. 1974; 80:49–53. PMID: 4359102.
Fig. 1
Contrast-enhanced T1-weighted coronal and axial magnetic resonance images of the brain show a slightly enhanced mass lesion in the left cavernous sinus (white arrows).
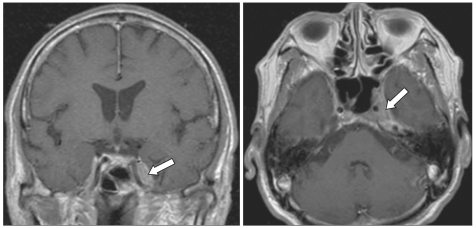
Fig. 2
18F-fluorodeoxyglucose (FDG) positron emission tomography shows intense FDG uptake in an extensive number of sites, including the abdominopelvic organs (small and large intestines [predominantly the ascending colon and rectum], peritoneum, omentum, mesentery, and the left adrenal gland), the skeletal system (maxilla, mandible, vertebrae, sternum, ribs, clavicles, scapulae, pelvic bones, humeri, and femurs), the left pleura, the bilateral skull base, and the left cavernous sinus.
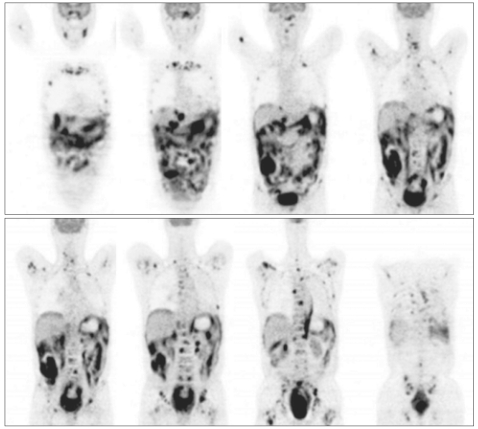
Fig. 3
(A) Tumor cells diffusely infiltrate the intestinal wall and are large lymphoid cells with oval to round, vesicular nuclei and scanty cytoplasm (H&E, ×400). (B) In addition, theses tumor cells were strongly reactive for a B-cell marker, CD20 (×400). (C) Ki-67 immunostaining revealed a high proliferation index (ki-67=90%).
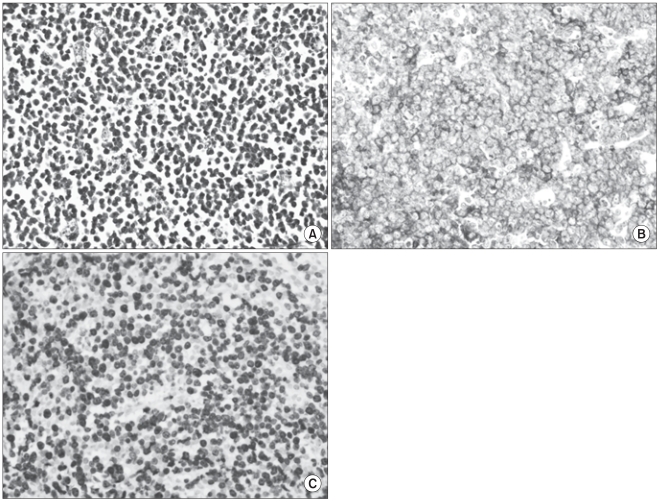
Table 1
Painful ophthalmoplegia (with/without numb chin syndrome) as a presenting symptom of brain metastasis: review of reported cases




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download