Abstract
This is a case report about benign metastasizing leiomyoma with multiple lymph node metastasis. A 34-year-old woman received an abdominal myomectomy for a suspicious leiomyoma. On the pathology report, atypical leiomyoma was suspected. Due to the suspicion of multiple lymph node metastasis on pelvis computed tomography (CT) 1 year after the operation, she was transferred to the Samsung Medical Center on October, 2009 for further work up. According to original slide review, it was determined to be a benign leiomyoma with a mitotic count <5/10 high-power fields, little cytological atypia and no tumor cell necrosis. Additional immunostaining was done. Multiple lymph node metastasis and a small lung nodule were identified on positron emission tomogarphy-CT and chest CT. Extensive debulking surgery and diagnostic video-assisted thoracoscopic surgery (VATS) wedge resection were subsequently done. Metastatic lesions were reported to have a histology similar to that of the original mass. VATS right upper lobectomy with mediastinal lymph node dissection was performed because of the pathology result of VATS (adenocarcinoma). She started taking an aromatase inhibitor (Letrozole®) and there was no evidence of recurrence of disease on an imaging study and no post-operative complications until recently.
Uterine leiomyomas are the most common benign gynecological tumor of women of reproductive age. Rarely, it shows unusual growth patterns with associated extrauterine smooth muscle deposits that seem to be derived from a benign uterine leiomyoma (benign metastasizing leiomyoma, BML). Hendrickson and Kempson [1] defined BML as a muscle tumor in association with one or more smooth muscle tumors of the uterus and without evidence of any extrauterine primary site. The risk factors, related etiology and clinical behaviors have been poorly studied. Lung is known to be the most common metastatic site. Pulmonary metastases are usually detected by chest radiographs, years after hysterectomy [2]. Except for lung, the extrauterine sites that these tumors can localize to are skin, pelvis, abdomen, muscle, greater omentum, inferior vena cava, right atrium, brain and bones [3]. However, multiple metastases to lymph nodes in BML are rare and studies about prognosis and treatment of BML with multiple lymph node metastasis are few. Here, we report a case of multiple BML in the lymph nodes. Clinical considerations are discussed.
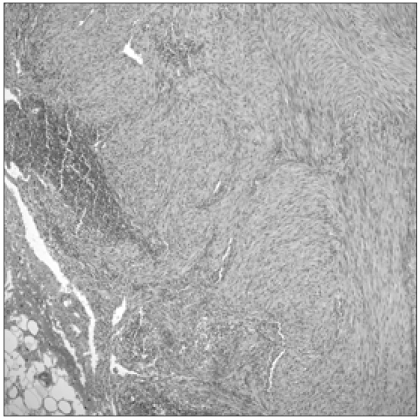
A 34-year-old women with no medical history (parity 1-0-0-1; normal spontaneous vaginal delivery at 2001, never a smoker) received an abdominal myomectomy for a palpable 8 cm abdominal mass with suspicion of leiomyoma by ultrasonography at a local clinic in July 2008. On pathology, it was found to be suspicious of cellular myoma or a smooth muscle tumor with uncertain malignant potential. She was then referred to the nearby university hospital. On pathology review, she was diagnosed as having a mitotically active leiomyoma and malignant potential. Due a suspicion of multiple lymph node metastases on pelvis computed tomography (CT) 1 year after the operation, she was transferred to the Samsung Medical Center (October 2009) for further work up. On pelvis CT, there was enlargement of multiple lymph nodes around the iliac chain and aorta. An original pathology slide review (Fig. 1) and positron emission tomogarphy (PET)-CT were done. According to the original slide review, it was a benign leiomyoma with a mitotic count <5/10 high-power fields (HPF), little cytological atypia and no tumor cell necrosis. Additional immunostaining indicated that smooth muscle actin and desmin were positive and CD10 was negative in the tumor cells. Tumor markers (TA-4, cancer antigen [CA]-125, CA-19-9, carcinoembryonic antigen) marked all normal. Abnormal fluorodeoxyglucose uptakes with the suspicion of paraaortic, aortocaval, precaval, external iliac lymph node and uterine body metastases were found on PET-CT. Incidentally, a 9 mm ground-glass opacity lesion at the right apical lung showed faint FDG uptake. Adenocarcinoma with bronchioloalveolar carcinoma rather than metastatic leiomyoma was suspected. An additional chest CT also revealed it to be a primary lung cancer. In December 2009 extensive debulking surgery including total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and paraaortic lymph node dissection to the level of the left renal vein, and gonadal lymph node dissection were done. Grossly, there were no definite mass-forming lesions in the uterus, and all the lymph nodes stated above were palpable and enlarged to about 2 to 3 cm in size. Pathology for an ill-defined residual intrauterine lesion was also a benign leiomyoma with mitosis <5/10 HPF, little cytological atypia and no tumor necrosis. Metastases to meso-ovarium/salpinx, pelvic lymph node, inferior mesenteric artery lymph node, para-aortic lymph node (Fig. 2), gonadal vein and to the parametrium were confirmed on the final pathology report. Metastatic lesions were also reported to have similar histology with the original mass with a mitotic count <2/10 HPF, little cytological atypism and no necrosis. Clinicopathologically, BML was selected as a final diagnosis with discussion with our pathologists (COS and GHA). On immunohistochemical staining, cells were all positive for estrogen/progesterone receptors. Video-assisted thoracoscopic surgery right upper lobectomy with mediastinal lymph node dissection for solitary lung nodule was also done (January 2010). On tumor-node-metastases staging, the patient was pT1aN0M0 (stage 1B) and it was consistent with a well-differentiated adenocarcinoma. The patient was discharged from the hospital without any early post-operative complications. She started taking an aromatase inhibitor (Letrozole® 2.5 mg daily, Femara, Novartis, Basel, Switzerland). In the 4 month follow-up of our patient, she had no clinical, biochemical or radiological evidence of recurrence disease or distant metastases. Her current general condition is satisfactory without any post-operative complications. However, long-term follow-up is required for assessment of disease recurrence and distant metastases.
BML is a relatively rare disease. It is considered to be benign histologically, but it occasionally metastasizes to distant sites such as lung, heart, inferior vena cava, retroperitoneal lymph nodes, muscles, and the bone (clinically malignant).
BML is usually characterized by uterine leiomyoma in young adulthood, with pulmonary metastasis occurring in the premenopausal period. Kayser et al. [4] reported that the mean interval between hysterectomy and the development of lung lesions was 24.9 years. Primary lung tumor in this case was incidentally detected on an imaging study, but since BML is known have metastases to lung most frequently, it is necessary to make a precise lung assessment during clinical follow up. There are a handful of reports about lymph node metastasis in BML. However, multiple pelvic and paraaortic lymph node metastases such as were present in this case are quite rare. Here, the authors report this rare case.
For an extrauterine lesion, a leiomyosarcoma has to be excluded. Pathologic and immunohistochemical studies to exclude other neoplasms are essential, especially when the metastatic site is uncommon. The metastatic potential of BML is not completely established. Previously reported cases of BML have included low-grade endometrial stromal sarcoma, which may play a significant role in the metastasis of benign uterine tumors. However some research using genetic analyses showed BML to be clonally derived from benign-appearing uterine leiomyomas [2].
There is no standard treatment guideline for BML currently. Reported treatment modalities include careful observation, surgical resection, hysterectomy and bilateral oophorectomy, progestins, aromatase inhibitor, and medical castration using luteinizing hormone-releasing hormone analogues [5]. A radical surgical resection, if possible, has been advocated as the primary treatment. Hormonal therapy has been suggested as the best option for unresectable metastatic disease. The rationale for the use of hormonal therapy is based on the presence of estrogen and progesterone receptors in both the primary tumor as well as in the metastatic tumors. Clinical evidence of a hormonal influence was supported by the fact that the pulmonary nodules diminish in size following menopause, during pregnancy and after the withdrawal of hormonal contraception. This is further supported by the beneficial effects of bilateral oophorectomy [6]. Depending on the locations of the metastases and the hormone receptor status, we believe that treatment should be individualized for each patient.
Long-term close surveillance is required in a BML patient for early detection of disease recurrence or distant metastases. Because of the limited therapeutic options, new drugs or new therapeutic modalities should be considered. In the future, studies with long-term follow-up may be helpful for their implication in clinical practice.
Acknowledgments
The authors would like to thank Dr. Geunghwan Ahn (Department of Pathology, University of California, San Francisco) for his experienced review of slides in this case.
References
1. Hendrickson MR, Kempson RL. Surgical pathology of the uterine corpus. Major Probl Pathol. 1979; 12:1–580. PMID: 537399.
2. Tietze L, Günther K, Hörbe A, Pawlik C, Klosterhalfen B, Handt S, et al. Benign metastasizing leiomyoma: a cytogenetically balanced but clonal disease. Hum Pathol. 2000; 31:126–128. PMID: 10665925.

3. Egberts JH, Schafmayer C, Bauerschlag DO, Jänig U, Tepel J. Benign abdominal and pulmonary metastasizing leiomyoma of the uterus. Arch Gynecol Obstet. 2006; 274:319–322. PMID: 16649038.

4. Kayser K, Zink S, Schneider T, Dienemann H, André S, Kaltner H, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Arch. 2000; 437:284–292. PMID: 11037349.

5. Funakoshi Y, Sawabata N, Takeda S, Hayakawa M, Okumura Y, Maeda H. Pulmonary benign metastasizing leiomyoma from the uterus in a postmenopausal woman: report of a case. Surg Today. 2004; 34:55–57. PMID: 14714229.

6. Abu-Rustum NR, Curtin JP, Burt M, Jones WB. Regression of uterine low-grade smooth-muscle tumors metastatic to the lung after oophorectomy. Obstet Gynecol. 1997; 89(5 Pt 2):850–852. PMID: 9166348.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download