1. Yancik R, Carbone PP, Patterson WB, Steel K, Terry WD, editors. Perspectives on prevention and treatment of cancer in the elderly. 1983. New York, NY: Raven Press.
2. Begg CB, Carbone PP. Clinical trials and drug toxicity in the elderly. The experience of the Eastern Cooperative Oncology Group. Cancer. 1983; 52:1986–1992. PMID:
6354419.

3. Gelman RS, Taylor SGt. Cyclophosphamide, methotrexate, and 5-fluorouracil chemotherapy in women more than 65 years old with advanced breast cancer: the elimination of age trends in toxicity by using doses based on creatinine clearance. J Clin Oncol. 1984; 2:1404–1132. PMID:
6512583.

4. Extermann M, Boler I, Reich R, Lyman GH, Brown RH, DeFelice J, et al. The CRASH score (Chemotherapy Risk Assessment Scale for High-Age Patients): Design and validation. Proc Annual Meeting Am Soc Clin Oncol. 2010. Chicago: IL: –636s.
5. Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross C, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective 500 patients multicenter study. Proc Annual Meeting Am Soc Clin Oncol. 2010. –636s.
6. Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007; 14:13–22. PMID:
17242667.

7. Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009; 27:3297–3302. PMID:
19487376.

8. Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003; 21:433–440. PMID:
12560431.

9. Extermann M, Albrand G, Chen H, Zanetta S, Schonwetter R, Zulian GB, et al. Are older French patients as willing as older American patients to undertake chemotherapy? J Clin Oncol. 2003; 21:3214–3219. PMID:
12874269.

10. Menzin J BL, Karsten V, Cahill AL, Earle CE. Effects of initial treatment on survival among elderly AML patients: findings from the SEER-Medicare database. Blood. 2006; 108:1973. (abstr).
11. Janssen-Heijnen ML, Gondos A, Bray F, Hakulinen T, Brewster DH, Brenner H, et al. Clinical relevance of conditional survival of cancer patients in europe: age-specific analyses of 13 cancers. J Clin Oncol. 2010; 28:2520–2528. PMID:
20406936.

12. Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009; 113:4179–4187. PMID:
19008455.

13. Bouchardy C, Rapiti E, Fioretta G, Laissue P, Neyroud-Caspar I, Schäfer P, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003; 21:3580–3587. PMID:
12913099.

14. Andersen SL, Terry DF, Wilcox MA, Babineau T, Malek K, Perls TT. Cancer in the oldest old. Mech Ageing Dev. 2005; 126:263–267. PMID:
15621206.

15. Extermann M, Boler I, Blair J, O'Neill E, Crane EJ, Balducci L, et al. Prevalence of multiple cancers in Floridian patients aged 70 years and older. Crit Rev Oncol Hematol. 2006; 60:S27.
16. Krach C. Centenarians in the United States, 1990. 1999. US Census Bureau.
17. Audisio RA, Ramesh H, Longo WE, Zbar AP, Pope D. Preoperative assessment of surgical risk in oncogeriatric patients. Oncologist. 2005; 10:262–268. PMID:
15821246.

18. Extermann M, Chen H, Cantor AB, Corcoran MB, Meyer J, Grendys E, et al. Predictors of tolerance to chemotherapy in older cancer patients: a prospective pilot study. Eur J Cancer. 2002; 38:1466–1473. PMID:
12110492.
19. Overcash J, Extermann M, Parr J, Perry J, Balducci L. Validity and reliability of the FACT-G scale for use in the older person with cancer. Am J Clin Oncol. 2001; 24:591–596. PMID:
11801761.

20. Monfardini S, Aversa SM, Zoli V, Salvagno L, Bianco A, Bordonaro R, et al. Vinorelbine and prednisone in frail elderly patients with intermediate-high grade non-Hodgkin's lymphomas. Ann Oncol. 2005; 16:1352–1358. PMID:
15857841.

21. Maione P, Perrone F, Gallo C, Manzione L, Piantedosi F, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005; 23:6865–6872. PMID:
16192578.

22. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007; 25:1824–1831. PMID:
17488980.

23. Freyer G, Geay JF, Touzet S, Provencal J, Weber B, Jacquin JP, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005; 16:1795–1800. PMID:
16093275.

24. Firat S, Bousamra M, Gore E, Byhardt RW. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002; 52:1047–1057. PMID:
11958901.

25. Firat S, Byhardt RW, Gore E. Radiation Therapy Oncology Group. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Int J Radiat Oncol Biol Phys. 2002; 54:357–364. PMID:
12243808.

26. Callen LJ VP, Overcash J, Boulware D, Extermann M. Survival and patterns of care in older cancer patients with cognitive impairment. 2004. San Francisco, CA:
27. Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002; 20:494–502. PMID:
11786579.

28. McCorkle R, Strumpf NE, Nuamah IF, Adler DC, Cooley ME, Jepson C, et al. A specialized home care intervention improves survival among older post-surgical cancer patients. J Am Geriatr Soc. 2000; 48:1707–1713. PMID:
11129765.
29. Extermann M, Meyer J, McGinnis M, Crocker TT, Corcoran MB, Yoder J, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol. 2004; 49:69–75. PMID:
14734156.

30. Extermann M, Green T, Tiffenberg G, Rich CJ. Validation of the Senior Adult Oncology Program (SAOP) 2 screening questionnaire. Crit Rev Oncol Hematol. 2009; 69:185.
31. Kenis C, Geeraerts A, Braesl T, Milisen K, Flamaing J, Wielders H. The Flemish version of the Triage Risk Screening Tool (TRST): a multidimensional short screening tool for the assessment of elderly patients. Crit Rev Oncol Hematol. 2006; 60:S31.
32. Mohile SG, Bylow K, Dale W, Dignam J, Martin K, Petrylak DP, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007; 109:802–810. PMID:
17219443.

33. Overcash JA, Beckstead J, Moody L, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA) for use in the older cancer patient as a prescreen: scoring and interpretation. Crit Rev Oncol Hematol. 2006; 59:205–210. PMID:
16904902.

34. Kenis C, Schuermans H, Van Custem E, Verhoel G, Vansteenkiste J, Vergote I, et al. Screening for a geriatric risk profile in older cancer patients: a comparative study of the predictive validity of three screening tools. Crit Rev Oncol Hematol. 2009; 72:S22.
35. Hitz F, Mey U, Clough-Gorr KM. Results from a pilot study of a brief Cancer-Specific Geriatric Assessment (CGA) tool for use in clinical trials in older cancer patients. Crit Rev Oncol Hematol. 2009; 72:S20–S21.
36. Johnson D, Balducci L, Extermann M, Crocker T, McGinnis M, Vranas P. The assessment of clinical resources in a aenior adult oncology program. 2006. In : European oncology nursing society meeting; Innsbruck, Austria.
37. Extermann M. Schrijvers D, Aapro M, Zakotnik B, Audisio R, van Halteren H, Hurria A, editors. Evaluation of the senior cancer patient: comprehensive geriatric assessment and screening tools for the elderly. Handbook of Cancer in the Senior Patient. 2010. New York, London: Informa Healthcare;p. 13–21.

38. Houterman S, Janssen-Heijnen ML, Hendrikx AJ, van den Berg HA, Coebergh JW. Impact of comorbidity on treatment and prognosis of prostate cancer patients: a population-based study. Crit Rev Oncol Hematol. 2006; 58:60–67. PMID:
16213153.

39. Vulto AJ, Lemmens VE, Louwman MW, Janssen-Heijnen ML, Poortmans PH, Lybeert ML, et al. The influence of age and comorbidity on receiving radiotherapy as part of primary treatment for cancer in South Netherlands, 1995 to 2002. Cancer. 2006; 106:2734–2742. PMID:
16703598.

40. Prout GR Jr, Wesley MN, Yancik R, Ries LA, Havlik RJ, Edwards BK. Age and comorbidity impact surgical therapy in older bladder carcinoma patients: a population-based study. Cancer. 2005; 104:1638–1647. PMID:
16130136.
41. Christman K, Muss HB, Case LD, Stanley V. Chemotherapy of metastatic breast cancer in the elderly. The Piedmont Oncology Association experience [see comment]. JAMA. 1992; 268:57–62. PMID:
1608114.

42. Hurria A, Hurria A, Zuckerman E, Panageas KS, Fornier M, D'Andrea G, et al. A prospective, longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy. J Am Geriatr Soc. 2006; 54:1119–1124. PMID:
16866685.

43. Chen H, Cantor A, Meyer J, Beth Corcoran M, Grendys E, Cavanaugh D, et al. Can older cancer patients tolerate chemotherapy? A prospective pilot study. Cancer. 2003; 97:1107–1114. PMID:
12569613.
44. Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999; 341:2061–2067. PMID:
10615079.

45. Kemeny MM, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003; 21:2268–2275. PMID:
12805325.

46. Kornblith AB, Kemeny M, Peterson BL, Wheeler J, Crawford J, Bartlett N, et al. Survey of oncologists' perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer. 2002; 95:989–996. PMID:
12209681.

47. Kimmick G, Kornblith A, Mandelblatt J, Peterson B, Johnson J, Wheeler J, et al. A randomized controlled trial of an educational program to improve accrual of older persons to cancer treatment protocols: CALGB 360001. 2004. In : Journal of Clinical Oncology, 2004 ASCO Annual Meeting Proceedings;
48. Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003; 21:1383–1389. PMID:
12663731.

49. Tirelli U, Errante D, Van Glabbeke M, Teodorovic I, Kluin-Nelemans JC, Thomas J, et al. CHOP is the standard regimen in patients > or = 70 years of age with intermediate-grade and high-grade non-Hodgkin's lymphoma: results of a randomized study of the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Study Group. J Clin Oncol. 1998; 16:27–34. PMID:
9440719.
50. Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005; 23:5027–5033. PMID:
15955905.

51. Balducci L, Lyman GH. Patients aged > or = 70 are at high risk for neutropenic infection and should receive hemopoietic growth factors when treated with moderately toxic chemotherapy. J Clin Oncol. 2001; 19:1583–1585. PMID:
11230505.
52. Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006; 24:3187–3205. PMID:
16682719.

53. Gridelli C. The ELVIS trial: a phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer. Elderly Lung Cancer Vinorelbine Italian Study. Oncologist. 2001; 1(Suppl 6):4–7. PMID:
11181997.
54. Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003; 95:362–372. PMID:
12618501.

55. Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000; 18:2529–2536. PMID:
10893283.

56. Quoix E, Oster J, Westeel V, Pichon E, Zalcman G, Baudrin L, et al. Weekly paclitaxel combined with monthly carboplatin versus single-agent therapy in patient age 70-89: IFCT-0501 randomized phase III study in advanced non-small cell lung cancer. 2010. In : Proc Am Soc Clin Oncol; –5s.
57. Jatoi A, Hillman S, Stella P, Green E, Adjei A, Nair S, et al. Should elderly non-small-cell lung cancer patients be offered elderly-specific trials? Results of a pooled analysis from the North Central Cancer Treatment Group. J Clin Oncol. 2005; 23:9113–9119. PMID:
16361618.

58. Castiglione M, Gelber RD, Goldhirsch A. International Breast Cancer Study Group. Adjuvant systemic therapy for breast cancer in the elderly: competing causes of mortality. J Clin Oncol. 1990; 8:519–526. PMID:
2407812.
59. Fargeot P, Bonneterre J, Roché H, Lortholary A, Campone M, Van Praagh I, et al. Disease-free survival advantage of weekly epirubicin plus tamoxifen versus tamoxifen alone as adjuvant treatment of operable, node-positive, elderly breast cancer patients: 6-year follow-up results of the French adjuvant study group 08 trial. J Clin Oncol. 2004; 22:4622–4630. PMID:
15505276.

60. Extermann M, Balducci L, Lyman GH. What threshold for adjuvant therapy in older breast cancer patients? J Clin Oncol. 2000; 18:1709–1717. PMID:
10764431.

61. Alibhai SM, Naglie G, Nam R, Trachtenberg J, Krahn MD. Do older men benefit from curative therapy of localized prostate cancer? J Clin Oncol. 2003; 21:3318–3327. PMID:
12947068.

62. Mandelblatt J, Yabroff KR, Lawrence W, Yi B, Orosz G, Bloom HG, et al. Screening mammography in elderly women. Research on Breast Cancer in Older Women Consortium. JAMA. 2000; 283:3202–3203. author reply 3204. PMID:
10866863.
63. Kerlikowske K, Salzmann P, Phillips KA, Cauley JA, Cummings SR. Continuing screening mammography in women aged 70 to 79 years: impact on life expectancy and cost-effectiveness. JAMA. 2000; 282:2156–2163. PMID:
10591338.
64. Anisimov VN. The relationship between aging and carcinogenesis: a critical appraisal. Crit Rev Oncol Hematol. 2003; 45:277–304. PMID:
12633840.

65. Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005; 120:513–522. PMID:
15734683.

66. Ershler WB. The influence of an aging immune system on cancer incidence and progression. J Gerontol. 1993; 48:B3–B7. PMID:
8418136.

67. Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005; 5:655–662. PMID:
16056261.

68. Gravekamp C, Kim SH, Castro F. Cancer vaccination: manipulation of immune responses at old age. Mech Ageing Dev. 2009; 130:67–75. PMID:
18561984.

69. Kennedy B, editor. Cancer Care in the Older Population. 2003. Alexandria, VA: American Society of Clinical Oncology.
70. Balducci L, Lyman GH, Ershler WB, Extermann M, editors. Comprehensive Geriatric Oncology. 2004. 2nd ed. London & New York: Taylor & Francis.
71. Schrijvers D, Aapro M, Zakotnik B, Audisio R, van Halteren H, Hurria A. Handbook of Cancer in the Senior Patient. 2010. New York, London: Informa Healthecare.
72. Hurria a, Balducci L. Geriatric Oncology. 2009. Dordrecht: Springer.
73. Balducci L, Ershler W, de Gaetano G. Blood Disorders in the Elderly. 2008. Cambridge: Cambridge University Press.
74. Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, et al. Use of Comprehensive Geriatric Assessment in older cancer patients. Recommendations from the Task Force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005; 55:241–252. PMID:
16084735.
75. Surbone A, Kagawa-Singer M, Terret C, Baider L. The illness trajectory of elderly cancer patients across cultures: SIOG position paper. Ann Oncol. 2007; 18:633–638. PMID:
17028242.

76. Extermann M. Studies of comprehensive geriatric assessment in patients with cancer. Cancer Control. 2003; 10:463–468. PMID:
14652522.

77. Ingram SS, Seo PH, Martell RE, Clipp EC, Doyle ME, Montana GS, et al. Comprehensive assessment of the elderly cancer patient: the feasibility of self-report methodology. J Clin Oncol. 2002; 20:770–775. PMID:
11821460.

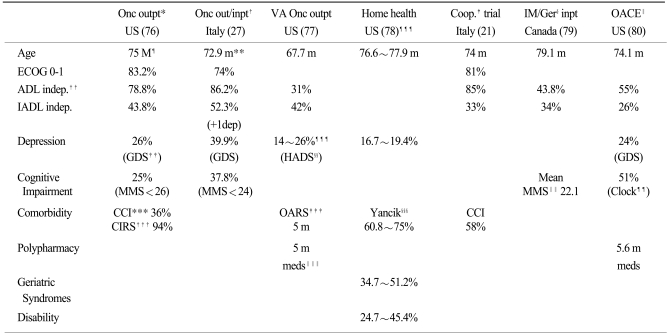
78. Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006; 24:2304–2310. PMID:
16710028.

79. Retornaz F. Personnal Communication. VIIIe Congres international francophone de gérontologie et gériatrie. 2006. Québec, Canada:
80. Flood KL, Carroll MB, Le CV, Ball L, Esker DA, Carr DB. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006; 24:2298–2303. PMID:
16710027.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download