Abstract
Trichilemmal carcinoma (TC) is an uncommon cutaneous neoplasm that develops from the external root sheath of the hair follicle. It is considered to be a low-grade carcinoma with low metastatic potential. Local recurrence and metastasis are rare after surgical excision. We report here on a case of metastatic TC in the skin over the thigh, and this tumor was treated with cisplatin and cyclophosphamide combination chemotherapy.
Go to : 
Trichilemmal carcinoma (TC) is a rare malignant tumor that develops from the external root sheath of the hair follicle. It usually is found in the skin of the face or ears of elderly women on areas that are exposed to the sun, and TC generally has an indolent clinical course (1,2). This condition is usually curable after a wide excision (2). TC must be differentiated from other skin abnormalities such as squamous cell carcinoma, basal cell carcinoma, keratoacanthoma, and invasive Bowen's disease (3-5). TC is a low-grade carcinoma with a low metastatic potential (6). Surgical excision is considered to be the first choice for curative treatment (7,8), and no other reliable treatment strategies for TC have yet been established (9).
We report here on a case of metastatic TC involving the skin over the thigh and this was treated with cisplatin and cyclophosphamide combination chemotherapy.
Go to : 
A 63-year-old woman presented with a mass lesion in the left thigh in September 2006. The medical history and family history were unremarkable. The lesion was 5×5×4 cm in size, with irregular margins and superficial erosion. The entire lesion was removed by performing wide surgical excision and the resection margin was free of tumor. The final diagnosis was TC, and the tumor was composed of several epithelial aggregations arranged in various growth patterns, including solid, lobular and trabecular patterns (Fig. 1). In addition, the skin biopsy revealed that the specimen included neoplastic cells with abundant, clear cytoplasm, and the cells showed prominent atypia and high mitotic activity (Fig. 2).
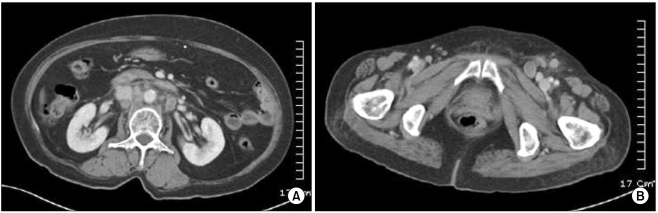
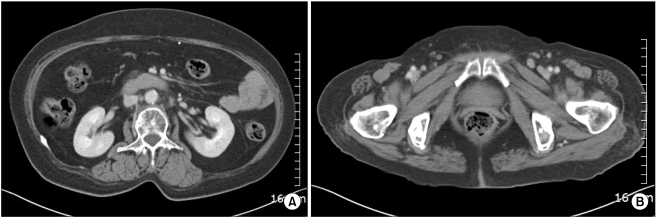
Two years later, the patient was hospitalized with swelling of the left lower leg and enlargement of the left inguinal lymph nodes. The blood pressure was 140/90 mmHg, the pulse rate was 78 bpm, the respiratory rate was 20 bpm and the body temperature was 37.4℃. Examination of the head and neck was unremarkable, no abnormality was found on the examination of the chest and no hepatosplenomegaly or mass was detected in the abdomen. The complete blood count showed a hemoglobin level of 12.6 g/dL, a hematocrit of 35.8%, a white blood cell count 6,490/µL and a platelet count of 333,000/µL. The sodium, potassium and calcium levels were all normal. An excisional biopsy was performed on an enlarged inguinal lymph node and the results demonstrated metastatic neoplastic cells with prominent atypia (Fig. 3). The histological features of this specimen and the initial specimen removed from the left thigh were similar. Moreover, the abdomino-pelvic CT scan showed multiple enlarged lymph nodes of variable size at the aortocaval, paraaortic and portocaval regions (Fig. 4). Therefore, metastatic TC was diagnosed based on the findings of the biopsy and the CT scan.
The patient was treated with four cycles of cisplatin and cyclophosphamide combination chemotherapy and a partial remission was achieved. There were no remarkable complications during chemotherapy. The chemotherapy was discontinued due to refusal by the patient and her family. Six months later, the patient was admitted with a cough, dyspnea and orthopnea. There were multiple pleural lesions, pericardial effusion, lymphangitic metastasis and osteoblastic lesions of the bony thorax on the thoracic CT scan, and lesions were also seen on the abdomino-pelvic CT scan. The patient received conservative treatment for one month and then she died in April 2009.
Go to : 
TC is an unusual malignant lesion. It originates from the hair follicle cells, and the differential diagnosis includes the other skin carcinomas (3-5). The common sites include the scalp, forehead and neck, which are sun-exposed areas (1). Women over 40 years of age are the most commonly affected patients. Grossly, the tumors have been described as exophytic, ulcerated, polypoid or nodular lesions that may be keratotic (1). Although the histological features suggest an intermediate to high grade malignancy, TC usually has an indolent clinical course (1). Recurrence or metastasis of this neoplasm has seldom been reported in the medical literature (10-12). Surgery is considered the treatment of choice for TC and periodic surveillance without adjuvant therapy is generally sufficient (4).
The patient reported here had local recurrence and paraaortic lymph node metastasis despite of the tumor excision two years ago. We thought that this fact could be explained by an initial inappropriate excision. Although the margin of resection was clear, we could not exclude the possibility that some cancer cells remained. It was also possible that the patient already had subclinical inguinal and abdominal lymph node metastasis when she was diagnosed with TC two years ago. The surgeon at a local clinic did not perform a metastatic work-up at the time of excision.
There is no consensus on the optimal treatment for TC with metastasis. There is no established chemotherapy regimen for metastatic TC reported in the medical literature. However, we found that Hayashi et al. administered chemotherapy (cisplatin, adriamycin, vindesine: CAV treatment) similar to the regimen used for highly advanced cases of squamous cell carcinoma (9,13).
The patient reported on here received four cycles of chemotherapy (cyclophosphamide 400 mg/m2, cisplatin 56 mg/m2: CP treatment). This treatment was effective and it resulted in a partial remission with a decreased size of the abdominal lymph nodes being observed on serial abdomino-pelvic CT scans. However, the CP treatment was not curative, but rather, it controlled the tumor growth. The chemotherapy regimen used for advanced squamous cell carcinoma, based on the similar cell cytology of the two tumors, might also be effective against metastatic TC.
Although TC has a low metastatic potential and it does not usually recur, any long term follow-up data has not been collected on the patients with this neoplasm. If tumor progression or metastasis is confirmed, then systemic chemotherapy should be considered to control the disease.
Go to : 
References
1. Reis JP, Tellechea O, Cunha MF, Baptista AP. Trichilemmal carcinoma: review of 8 cases. J Cutan Pathol. 1993; 20:44–49. PMID: 8468416.

2. Maize JC, Snider RL. Nonmelanoma skin cancers in association with seborrheic keratoses. Clinicopathologic correlations. Dermatol Surg. 1995; 21:960–962. PMID: 7582834.
3. Misago N, Tanaka T, Kohda H. Trichilemmal carcinoma occurring in a lesion of solar keratosis. J Dermatol. 1993; 20:358–364. PMID: 8349925.

4. Ko T, Tada H, Hatoko M, Muramatsu T, Shirai T. Trichilemmal carcinoma developing in a burn scar: a report of two cases. J Dermatol. 1996; 23:463–468. PMID: 8772025.
5. Chan KO, Lim IJ, Baladas HG, Tan WT. Multiple tumour presentation of trichilemmal carcinoma. Br J Plast Surg. 1999; 52:665–667. PMID: 10658141.

6. Oyama N, Kaneko F. Trichilemmal carcinoma arising in seborrheic keratosis: a case report and published work review. J Dermatol. 2008; 35:782–785. PMID: 19239559.

7. Amaral AL, Nascimento AG, Goellner JR. Proliferating pilar (trichilemmal) cyst. Report of two cases, one with carcinomatous transformation and one with distant metastases. Arch Pathol Lab Med. 1984; 108:808–810. PMID: 6548121.
8. Weiss J, Heine M, Grimmel M, Jung EG. Malignant proliferating trichilemmal cyst. J Am Acad Dermatol. 1995; 32:870–873. PMID: 7722047.

9. Hayashi I, Harada T, Muraoka M, Ishii M. Malignant proliferating trichilemmal tumour and CAV (cisplatin, adriamycin, vindesine) treatment. Br J Dermatol. 2004; 150:156–157. PMID: 14746636.

10. Wong TY, Suster S. Tricholemmal carcinoma. A clinicopathologic study of 13 cases. Am J Dermatopathol. 1994; 16:463–473. PMID: 7528473.
11. Garrett AB, Azmi FH, Ogburia KS. Trichilemmal carcinoma: a rare cutaneous malignancy: a report of two cases. Dermatol Surg. 2004; 30:113–115. PMID: 14692940.

12. Swanson PE, Marrogi AJ, Williams DJ, Cherwitz DL, Wick MR. Tricholemmal carcinoma: clinicopathologic study of 10 cases. J Cutan Pathol. 1992; 19:100–109. PMID: 1597565.

13. Ikegawa S, Saida T, Obayashi H, Sasaki A, Esumi H, Ikeda S, et al. Cisplatin combination chemotherapy in squamous cell carcinoma and adenoid cystic carcinoma of the skin. J Dermatol. 1989; 16:227–230. PMID: 2551943.

Go to : 




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download