Abstract
Purpose
The aim of this study was to determine whether the ERCC1 expression is effective to predict the clinical outcomes of patients with advanced gastric cancer (AGC) and who were treated with cisplatin-based first-line chemotherapy.
Materials and Methods
A total of 89 measurable AGC patients received cisplatin and capecitabine, with or without epirubicin, as a part of a randomized phase II study. Patients were included for the current molecular analysis if they had received two or more cycles of chemotherapy, their objective tumor responses were measured and if their paraffin-embedded tumor samples were available. The ERCC1 expression was examined by performing immunohistochemical (IHC) staining, and the patients were divided into two groups (positive or negative) according to the presence of IHC staining of the tumor cell nuclei.
Results
Of the 32 eligible patients, 21 patients (66%) had tumor with a positive expression of ERCC1 and the remaining 11 patients had tumor with a negative ERCC1-expression. The ERCC1-negative patients achieved a higher response rate than that of the ERCC1-positive patients (44% vs. 28%, respectively), although the difference was not statistically significant (p=0.42). The median survival time for the all patients was 14.6 months (95% CI: 13.6 to 15.6 months). The one-year survival rate was similar for the ERCC1-negative patients (61%) and the ERCC1-positive patients (70%).
Conclusion
In the current study, the tumor ERCC1 expression by IHC staining could not predict the clinical response or survival of AGC patients who were treated with cisplatin-based first-line chemotherapy. The ERCC1 protein expression does not appear to be a useful tool for the selection of tailored chemotherapy for these patients.
Gastric cancer is the most frequently occurring malignancy in Korea, and it is one of the main causes of cancer death (1). Fluropyrimidine-based chemotherapy has shown a significant survival advantage for patients with advanced gastric cancer (AGC) when compared with the best supportive care in randomized clinical trials (2). Specifically, the apparent synergy between fluoropyrimidines and cisplatin has led to the widespread use of regimens that combine these agents.
Platinum asserts its cytotoxicity through disrupting double stranded DNA in cells by forming intra-strand adducts that inhibit DNA replication. Nucleotide excision repair (NER) is one of several DNA repair pathways for correcting the abnormal DNA structures that arise from DNA damage, replication errors or recombination processes (3). NER is associated with resistance to platinum-based chemotherapy (4). The excision repair cross-complementation group 1 (ERCC1) is a rate-limiting DNA repair protein in the nucleotide excision repair pathway that recognizes and removes cisplatin-induced DNA adducts (5-8). ERCC1 is also important for repairing interstrand cross-links in the DNA and in recombination processes (9-11).
Olaussen et al. (12) suggested that patients with completely resected non-small-cell lung cancer and ERCC1-negativity derived substantial benefit from adjuvant cisplatin-based chemotherapy, as compared with the patients with ERCC1-positive tumors. Subsequently, several studies have suggested that ERCC1 may serve as an effective biomarker for chemosensitivity and survival in patients with non-small-cell lung cancer and colorectal cancer and who are treated with platinum-based regimens (13-15).
Recent studies have been done on tailored therapy by selecting the patients who are likely to respond to a particular chemotherapeutic regimen, and this may allow improved treatment efficacy while avoiding unnecessary treatment side effects. In an effort to investigate the role of the ERCC1 protein expression as a predictive marker with respect to cisplatin-based chemotherapy for AGC, we conducted an exploratory analysis of the archived tumor tissues that were obtained within a prospective phase II study. The study population of that prospective phase II study consisted of chemotherapy-naïve AGC patients who were treated with cisplatin plus capecitabine combination regimens (16).
This is a retrospective, exploratory analysis of the patients who were drawn from a prospective phase II study (16). To enter that clinical study, the patients were required to have histologically confirmed AGC. The patients had to be under 76 years of age and they had to have an adequate performance status (0 to 2 according to the Eastern Cooperative Oncology Group [ECOG] scale), measurable lesion(s) and adequate organ function. They received first-line therapy with cisplatin and capecitabine (CX), or epirubicin plus CX (ECX). Therapy was continued until disease progression, unacceptable toxicity or consent withdrawal. Further second-line chemotherapy could be administered at the discretion of the physicians who were in charge after first line chemotherapy failure. The clinical study included 89 patients with AGC and who were treated with CX (n=45) or ECX (n=44). Patients were included for the current molecular analysis if they received two or more cycles of chemotherapy with their objective responses being recorded, and if their paraffin-embedded tumor samples were available. The characteristics of the patients included in this analysis were comparable to those of the patients who were not included. At the time of analysis, there was no significant difference in survival between the patients with and without an ERCC1 expression status. The primary objective of this study was to determine whether the ERCC1 expression in the tumor samples of the consecutive AGC patients who were treated with cisplatin-based chemotherapy was associated with the clinical response rate (RR), the progression-free survival (PFS) and/or the overall survival (OS). All the tumor samples were collected for a routine histopathologic diagnosis before the start of therapy. All the patients provided us with a written informed consent form and this study was reviewed and approved by the Samsung Medical Center (Seoul, Korea) institutional review board.
The response was evaluated after every two cycles of therapy according to the Response Evaluation Criteria in Solid Tumors (RECIST), and the response was assessed by abdominopelvic CT or by the same tests that were initially used to stage the tumor. PFS was calculated from the day of registration to the date of progression, death or the last contact. OS was measured from the day of registration to death or the last contact date. The results of the 89 patients from the previous clinical study have been reported elsewhere (16).
Paraffin-embedded tumor material from the biopsy was cut into 2 µm-thick sections and placed onto glass slides. For immunohistochemical (IHC) staining, the slides were depafaffinized in xylene. Staining for ERCC1 was performed automatically using the Leica Bond Max immunostainer with Bond Polymer Refine Detection Kits and heat-induced epitope retrieval pH 8.0 (Bond max ER2 (EDTA) solution, Australia) for 15 min. The ERCC1 expression was analyzed using a mouse monoclonal antibody specific for the full-length human ERCC1-protein (Clone 8F1, 1 : 150, GeneTex, CA).
Protein staining was assessed semiquantitatively by a pathologist (KMK) who was kept "blind" to the clinical data. The ERCC1 nuclear and cytoplasmic immunoreactivity were evaluated semiquantitatively, and the percentage of positive tumor nuclei was calculated for each specimen at 400×. A biopsy with the cells showing positive nuclear staining was defined as positive.
The correlations between the IHC expression and the response to chemotherapy were examined using the Chi-square test or Fisher's exact test, as appropriate. Logistic regression analysis was used to calculate the odds ratios and their confidence intervals (CI). Additionally, the other known clinical factors (gender, the performance status, the chemotherapy regimens, the metastatic sites and the previous therapy) were included in the multivariate analysis. The Cox proportional hazards model (as stratified by the chemotherapy regimens: CX vs. ECX) was used to compare the PFS and OS between the patients with ERCC1-negative and positive tumor. The association between the ERCC1 status and the other clinical characteristics was tested for by chi-square tests.
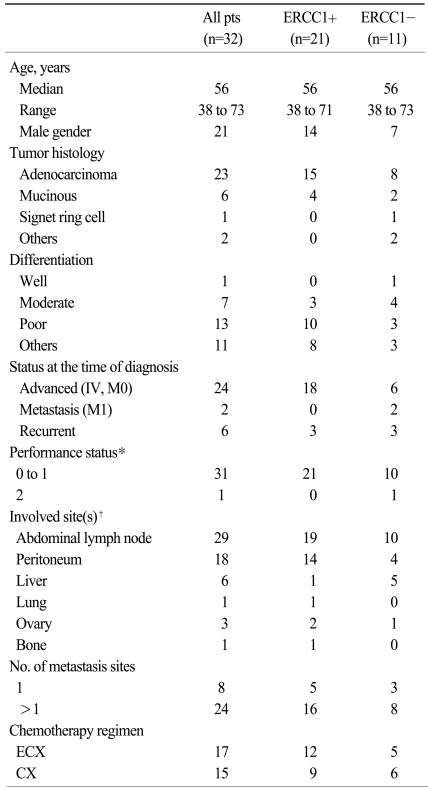
Of the 89 patients who were treated with either CX or ECX, 81 patients received at least two cycles of chemotherapy. Of these 81 patients, 32 patients provided tumor samples for ERCC1 analysis. The clinical characteristics of the eligible patients are listed in Table 1. Overall, 21 patients (66%) had tumor with a protein expression of ERCC1. The cisplatin-based chemotherapy was generally well tolerated. Although not specified in the protocol, second-line chemotherapy was offered to 18 patients after failure: 8 for the ERCC1-negative patients and 10 for the ERCC-1 positive patients.
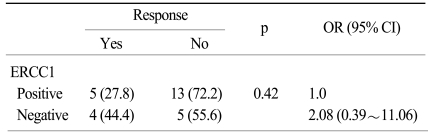
Eleven of the 32 patients (34%) achieved an objective response. Although it was statistically insignificant, a higher RR to chemotherapy was noted for the patients with ERCC1-negative tumors as compared to those patients with ERCC1-positive tumors (44% vs. 28%, respectively; p=0.42). Besides ERCC1 positivity, another possible factor associated with the lack of optimal response was the ECOG performance status (36% for ECOG 0-1 vs. 0% for ECOG 2; p=0.01). The RR was not influenced by gender, age, the chemotherapy regimens, the histology or tumor differentiation, or a prior history of adjuvant treatment. Using a multiple logistic regression model for which we entered all the predictive factors as covariates, the ERCC1 status (odds ratio: 2.08) was not associated with the RR to chemotherapy (Table 2).
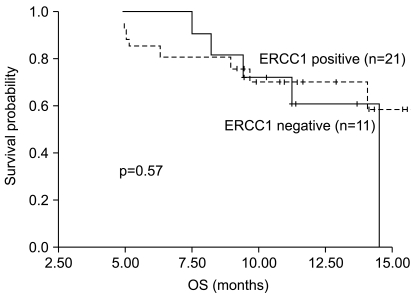
The median PFS and OS were 6.9 months (95% CI: 5.4 to 8.4 months) and 14.6 months (95% CI: 13.6 to 15.6 months), respectively. There was no statistically significant difference between the ERCC1 expression statuses and OS (p=0.57). The Kaplan-Meier OS curves are presented in Fig. 1 and they showed that the probability of survival at 12 months was 70% for the patients with ERCC1-positive tumors compared with 61% for the patients with ERCC1-negative ones.
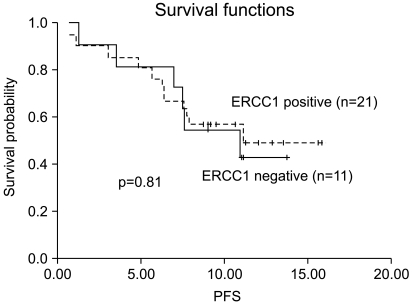
We also analyzed the association between the ERCC1 expression status and PFS. The respective PFS curves are presented in Fig. 2 and they showed that the probability of PFS at 6 months was 76% for the patients with ERCC1-positive tumors compared with 82% for the patients with ERCC1-negative tumors. Again, there was no statistically significant difference between the ERCC1 expression status and PFS (p=0.81). The Cox proportional hazards model failed to reveal any independent prognostic variables for PFS or OS.
Although the multi-drug combination chemotherapy regimens have failed to provide significantly better OS than fluoropyrimidine monotherapy (17), cisplatin-based combination regimens that are mostly composed of fluoropyrimidine and cisplatin have been widely used because monotherapy has only limited activity (2). We recently reported the results of a randomized phase II study that compared CX and ECX, which were given as first-line chemotherapy for AGC (16). Both regimens were tolerable and effective, but ECX did not prove to be superior to CX in terms of RR or PFS. In this current small, retrospective, exploratory analysis, the prognostic and predictive roles of the ERCC1 protein expression were evaluated in AGC patients who were treated with cisplatin-based first-line chemotherapy. The positivity of an ERCC1 protein expression resulted in a tendency for a lower RR, yet there were no correlations between the ERCC1 status and the clinical outcomes.
Several previous studies have suggested that the ERCC1 status is associated with RR, and the ERCC1 status is prognostic for cisplatinbased chemotherapy. Metzger et al. (18) have reported a statistically significant association between the ERCC1 mRNA levels and survival after cisplatin and fluorouracil chemotherapy for the patients with primary gastric cancer. Other studies have also found an association between a high ERCC1 mRNA level and the unfavorable clinical outcomes after cisplatin-based therapy for a variety of tumor types, including non-small cell lung cancer (12), cervical cancer (19), ovarian cancer (20) and colorectal cancer (21). The recent interest in the ERCC1 status as a possible predictor for a response to chemotherapy is largely derived from the studies that have been performed in a retrospective fashion. Although the ERCC1 status may be used to identify the patients who are most likely to benefit from chemotherapy, this only identifies a fraction of these patients. In the current study, a clinical response was also observed for the patients with ERCC1-positive tumors (28%) and there was no apparent reduction in the risk of disease progression or death in the subset of patients with ERCC1-negative tumors. While the current analysis was retrospectively done, the patients were selected from a prospective clinical study that was concerned with fluoropyrimidine and cisplatin combination chemotherapy.
There may be several reasons that could explain the discrepancies in the studies. The incidence of an ERCC1 protein expression was not consistent in the previous studies. The reason for the difference in the incidence of an ERCC1 expression can be explained, to some extent, by the different patient populations. Our result should be interpreted with caution because it represents only a small group of patients with AGC in this study and the majority of patients had unfavorable clinical characteristics: poorly-differentiated carcinoma in 41% of the patients and 75% had more than one metastatic site. Our study is limited due to a small sample size, but it only indicated that ERCC1-positive AGC could respond to cisplatin-based chemotherapy.
Moreover, the lack of a uniformly approved method for detecting the ERCC1 expression suggests that any conclusions must still be regarded as preliminary at best. There are different sensitivities and specificities between reverse transcriptase-polymerase chain reaction (RT-PCR) and the IHC staining for detecting the ERCC1 expression. The ERCC1 levels can be measured by performing RT-PCR on the intratumoral ERCC1 mRNA derived from paraffin-embedded or fresh-frozen tumor specimens. Most of the previous studies used the RT-PCR method, which is currently known to be the gold standard rather than IHC (18,21). Although real time RT-PCR is a highly sensitive and semiquantitative method, it is not always easy to obtain fresh tumor samples. Kwon et al. (22) suggested that IHC studies for ERCC1 may be useful to predict the clinical outcome of AGC patients who are treated with fluorouracil and oxaliplatin. Similarly, Wang et al. (23) proposed that the ERCC1 expression using IHC staining may serve as a biomarker for predicting the tumor response and the clinical outcomes, even though this was for Chinese patients with advanced non-small cell lung cancer. Further studies are needed that can explain the discrepancy between RT-PCR and IHC when using the ERCC1 gene as a predictive marker.
Another explanation for the current study's negative results is that the ERCC1 expression might not have a defined role as a predictive marker because cisplatin is not likely to play a major role in treating gastric cancer. Unlike lung cancer or ovarian cancer in which platinum is the standard of care, AGC patients are usually treated with fluoropyrimidine-based chemotherapy. Although combination chemotherapy regimens involving fluoropyrimidines and cisplatin are most commonly used for AGC patients, the role of cisplatin in the treatment of AGC is currently limited. In this study, we could not find a correlation between the clinical outcome and the expression of ERCC1. Furthermore, there might have been an unbalanced distribution of other genetic variants that may potentially interfere with the efficacy of chemotherapy, such as those genetic variants in the thymidylate synthase gene (22). While there was no relevant difference in the OS between the patients with or without an ERCC1 expression, it is possible that salvage treatment by second-line chemotherapy or even further chemotherapy after cisplatin failure could have influenced the survival results.
ERCC1 may be prognostic for the survival of cancer patients who are treated with platinum-based chemotherapy. However, it is currently unclear if a high ERCC1 expression is the direct cause of a poor prognosis or if it is just a marker for chemotherapy resistance. Interestingly, an ERCC1 expression has been shown to have paradoxical prognostic significance in a previous study. For the lung cancer patients who did not receive platinum-based chemotherapy, the patients with ERCC1-positive tumors had a better survival when compared with those with ERCC1-negative tumors (12,15). Besides cisplatin, other chemotherapy drugs may interfere with the ERCC1 expression. An in vitro study has shown that radiation activates the ERCC1 expression in gastrointestinal cancer cell lines, but not in the same cell lines that were treated with paclitaxel before radiation, and this suggested that paclitaxel may suppress the ERCC1 expression (24). In a clinical study that was conducted on ovarian cancer patients who were treated with cisplatin with or without paclitaxel (25), high levels of ERCC1 mRNA were associated with a greater risk of disease progression. However, for the patients treated with cisplatin plus paclitaxel, a high ERCC1 expression was not associated with a poor prognosis, and this suggested that paclitaxel may help to alleviate ERCC1-related platinum resistance. Among other antimetabolites, gemcitabine is an antimetabolite that may have an influence on the pool of nucleosides that is available for DNA repair, and so it may interact with the NER pathway.
Our analyses suggested that an ERCC1 expression is not perfect for determining the responsiveness of patients to cisplatin-based chemotherapy, although may be important. The ERCC1 expression did not appear to be a useful tool for the selection of tailored chemotherapy to treat patients with AGC. Given our findings, we question the validity of selecting patients who would benefit from cisplatin-based chemotherapy based on only the ERCC1 expression. Selecting patient in such a manner would lead to inappropriately turning away patients who would otherwise possibly benefit from platinum-based therapy, be it monotherapy or part of combination chemotherapy.
References
1. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide Cancer Incidence in Korea, 2003-2005. Cancer Res Treat. 2009; 41:122–131. PMID: 19809561.

2. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006; 24:2903–2909. PMID: 16782930.

4. Reed E. Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev. 1998; 24:331–344. PMID: 9861196.

5. Bepler G, Gautam A, McIntyre LM, Beck AF, Chervinsky DS, Kim YC, et al. Prognostic significance of molecular genetic aberrations on chromosome segment 11p15.5 in non-small-cell lung cancer. J Clin Oncol. 2002; 20:1353–1360. PMID: 11870179.

6. Mu D, Hsu DS, Sancar A. Reaction mechanism of human DNA repair excision nuclease. J Biol Chem. 1996; 271:8285–8294. PMID: 8626523.

8. Zamble DB, Mu D, Reardon JT, Sancar A, Lippard SJ. Repair of cisplatin--DNA adducts by the mammalian excision nuclease. Biochemistry. 1996; 35:10004–10013. PMID: 8756462.

9. De Silva IU, McHugh PJ, Clingen PH, Hartley JA. Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand cross-links in mammalian cells. Mol Cell Biol. 2000; 20:7980–7990. PMID: 11027268.

10. Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004; 24:5776–5787. PMID: 15199134.

11. Sargent RG, Meservy JL, Perkins BD, Kilburn AE, Intody Z, Adair GM, et al. Role of the nucleotide excision repair gene ERCC1 in formation of recombination-dependent rearrangements in mammalian cells. Nucleic Acids Res. 2000; 28:3771–3778. PMID: 11000269.

12. Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006; 355:983–991. PMID: 16957145.

13. Simon GR, Ismail-Khan R, Bepler G. Nuclear excision repair-based personalized therapy for non-small cell lung cancer: from hypothesis to reality. Int J Biochem Cell Biol. 2007; 39:1318–1328. PMID: 17600754.

14. Viguier J, Boige V, Miquel C, Pocard M, Giraudeau B, Sabourin JC, et al. ERCC1 codon 118 polymorphism is a predictive factor for the tumor response to oxaliplatin/5-fluorouracil combination chemotherapy in patients with advanced colorectal cancer. Clin Cancer Res. 2005; 11:6212–6217. PMID: 16144923.

15. Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007; 356:800–808. PMID: 17314339.
16. Yun J, Lee J, Park SH, Park JO, Park YS, Lim HY, et al. A randomised phase II study of combination chemotherapy with epirubicin, cisplatin and capecitabine (ECX) or cisplatin and capecitabine (CX) in advanced gastric cancer (AGC). Eur J Cancer. 2010; 46:885–891. PMID: 20060288.
17. Ohtsu A, Yoshida S, Saijo N. Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol. 2006; 24:2188–2196. PMID: 16682738.

18. Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, Hayashi K, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998; 16:309–316. PMID: 9440758.

19. Britten RA, Liu D, Tessier A, Hutchison MJ, Murray D. ERCC1 expression as a molecular marker of cisplatin resistance in human cervical tumor cells. Int J Cancer. 2000; 89:453–457. PMID: 11008208.

20. Codegoni AM, Broggini M, Pitelli MR, Pantarotto M, Torri V, Mangioni C, et al. Expression of genes of potential importance in the response to chemotherapy and DNA repair in patients with ovarian cancer. Gynecol Oncol. 1997; 65:130–137. PMID: 9103402.

21. Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001; 19:4298–4304. PMID: 11731512.
22. Kwon HC, Roh MS, Oh SY, Kim SH, Kim MC, Kim JS, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007; 18:504–509. PMID: 17322540.

23. Wang X, Zhao J, Yang L, Mao L, An T, Bai H, et al. Positive expression of ERCC1 predicts a poorer platinum-based treatment outcome in Chinese patients with advanced non-small-cell lung cancer. Med Oncol. 2009; (in press).

24. Toiyama Y, Inoue Y, Hiro J, Ojima E, Watanabe H, Narita Y, et al. The range of optimal concentration and mechanisms of paclitaxel in radio-enhancement in gastrointestinal cancer cell lines. Cancer Chemother Pharmacol. 2007; 59:733–742. PMID: 16957932.

25. Smith S, Su D, Rigault de la Longrais IA, Schwartz P, Puopolo M, Rutherford TJ, et al. ERCC1 genotype and phenotype in epithelial ovarian cancer identify patients likely to benefit from paclitaxel treatment in addition to platinum-based therapy. J Clin Oncol. 2007; 25:5172–5179. PMID: 18024864.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download