Abstract
Purpose
The aim of this study was to determine the prognosis of pN3 stage gastric cancer patients after they have undergone curative resection, and we also wanted to identify the prognostic factors according to the clinico-pathologic features.
Materials and Methods
Between January 2000 and December 2004, we retrospectively reviewed the medical records of the patients with histologically confirmed pN3 stage gastric cancer. They underwent both gastrectomy and lymphadenectomy with a curative aim. We categorized the pN3 stage patients into 2 groups; one with pN3 only (pN3M0) and the other with pN3 combined with M1 stage (pN3M1) that included peritoneal seeding, hepatic metastasis or para-aortic LN metastasis.
Results
Out of 467 patients with stage IV gastric adenocarcinoma who received surgery, 260 patients underwent curative resection and they were pathologically staged as N3. Among these 260 patients, 78 patients were classified as the pN3/M1 stage. For all the patients, the median follow-up period was 19 months (range: 1~108 months) and the median overall survival time was 16.2 months (95% CI, 14.1~18.3%). The 5-year survival rate of the pN3/M0 group was significantly higher than that of the pN3/M1 group (12.6% vs. 2.6%, respectively, p<0.0001). The identified predictor for a worse prognosis was an advanced T4 stage (HR: 3.38, 95% CI, 1.4~8.3, p=0.008) for the pN3 patients.
Conclusion
The survival for the pN3 gastric cancer patients after curative gastrectomy was significantly longer in the pN3/M0 group as compared to that of the pN3/M1 group. An advanced T stage was a predictor for a poor prognosis for the pN3 patients. Therefore, diverse treatment strategies for these heterogeneous pN3 gastric cancer patients are needed for improving their survival.
Gastric cancer is the fourth most common cancer worldwide (1). According to the global estimation-GLOBOCAN 2002, gastric cancer has the second and fourth highest mortality rate for men and women, respectively (2). The prognosis of gastric cancer patients is poor with a 5-year survival rate of approximately 20% (1,3). Surgical resection with a curative aim is the principal treatment for gastric cancer and the indications for surgical resection have been decided on based on the stage (4). The fifth edition of the American Joint Committee on Cancer (AJCC) cancer staging system has been modified with the main change of nodal staging which was based on the number of metastatic lymph nodes (5). When applying the fifth AJCC system, some studies have reported 23% of the patients migrated to another stage, including the patients who migrated to stage IV (5). Thus, there are variations in the survival rates even among the patients with the same stage IV gastric cancer. It is still controversial that these heterogeneous groups of patients are being treated with the same strategies according to the grouping as stage IV.
For the stage IV patients who cannot be cured, several studies have shown that resection may be beneficial in terms of survival (4). The benefit of surgery has been reported that the survival of patients with stage IV gastric cancer was significantly better with resection than with bypass procedures or laparotomy alone (6). In addition, it has also been reported that the patients with pN3/M0 gastric cancer showed a higher survival rate among the stage IV patients with other pathologic characteristics (7). It has been found that the 5-year survival rate of pN3M0 gastric cancer was 8.7%, which was slightly better than that of other stage IV gastric cancers (about 4.1%) (p=0.039) (7).
We hypothesize that the prognosis may be different among stage IV patients, and especially between the pN3 stage patients whose tumor was removed and the other stage IV gastric cancer patients with gross residual tumors. Therefore, the aim of this study was to determine whether the pN3 stage gastric cancer patients are a heterogeneous group with different prognoses among the stage IV patients. We also determined the prognostic factors of pN3 stage gastric cancer patients according to the clinico-pathologic features.
Between January 2000 and December 2004, among 467 patients with histopathologically diagnosed stage IV adenocarcinoma and who received surgical treatment at the Yonsei Cancer Center, Yonsei University Health System, we enrolled 260 patients who underwent surgery for primary gastric cancer and they were staged at the pN3 stage. The last follow up date was December 31 2008.
The pathology was confirmed as gastric adenocarcinoma according to endoscopic biopsy. Preoperative computed tomography was done to evaluate for possible metastatic disease in the abdominal organs and lymph nodes. All the enrolled patients had the following curative aim for their operative procedures: 1) total or subtotal gastrectomy depending on the location and 2) D2 or D3 lymphadenectomy. Curative resection was defined as having no remaining grossly visible tumor tissue and microscopically negative surgical margins on the sufficient margins around the tumor. A margin of 2 cm for expanding tumors and a 2~3 cm distal surgical margin for the pylorus were regarded as sufficient (8). M1 stage was defined in this study as metastasis with peritoneal seeding or hepatic metastasis or the presence of para-aortic lymph node metastasis.
Lymph node dissection followed the guideline of the Japanese Research Society for Gastric Cancer (JRSGC) (9). D2 lymphadenectomy was performed for all the Group 1 and Group 2 lymph nodes, and D3 lymphadenectomy was performed for all the Group 1, Group 2 and Group 3 lymph nodes. The retrieved lymph nodes were classified by the surgeons in the operation rooms and all the lymph nodes were inspected by light microscopy for metastasis. The pN3 stage was defined as metastasis in more than 15 regional lymph nodes according to the sixth edition of the UICC TNM classification.
The clinical and pathological features of the N3 patients were retrospectively reviewed. We then analyzed survival according to such factors as age, gender and tumor location, the type of gastrectomy, the gross features of the tumor, tumor size, tumor differentiation, the depth of invasion, Lauren classification, lymphatic invasion, vascular invasion and the number of retrieved and metastatic lymph nodes.
We subdivided the patients into two groups as the pN3/M0 group and the pN3/M1 group: 1) the pN3/M0 group was diagnosed as the pN3 stage only and they had no distant metastasis or para-aortic lymph node metastasis, 2) the pN3/M1 group was diagnosed as the pN3 stage with peritoneal metastasis, hepatic metastasis or para-aortic lymph node metastasis (Table 1).
The end point of this study was overall survival, and this was defined as the time from operation to death or to the last follow-up date. We analyzed the overall survival of the total patients and we compared survival of the pN3/M0 group with that of the pN3/M1 group. We also investigated the significant prognostic factors for all the cases of pN3 gastric cancer and for the two groups. We used X2-square or Fisher's exact tests, except for the comparisons of age and tumor size for which we used the Mann-Whitney U test. We calculated the probability of survival using the Kaplan-Meier method. The differences between survival curves were calculated by the log-rank test. The multivariate analysis was done using the Cox proportional hazard model, and the hazard ratios were calculated. A p-value of <0.05 was considered statistically significant.
Among the 260 gastric cancer patients who underwent gastrectomy and who were finally confirmed as being pN3 stage, there were 175 (67.3%) males and 85 (32.7%) females. The clinico-pathology features of the patients are shown in Table 1. The median age of the 260 patients was 59 years (range: 16~82 years).
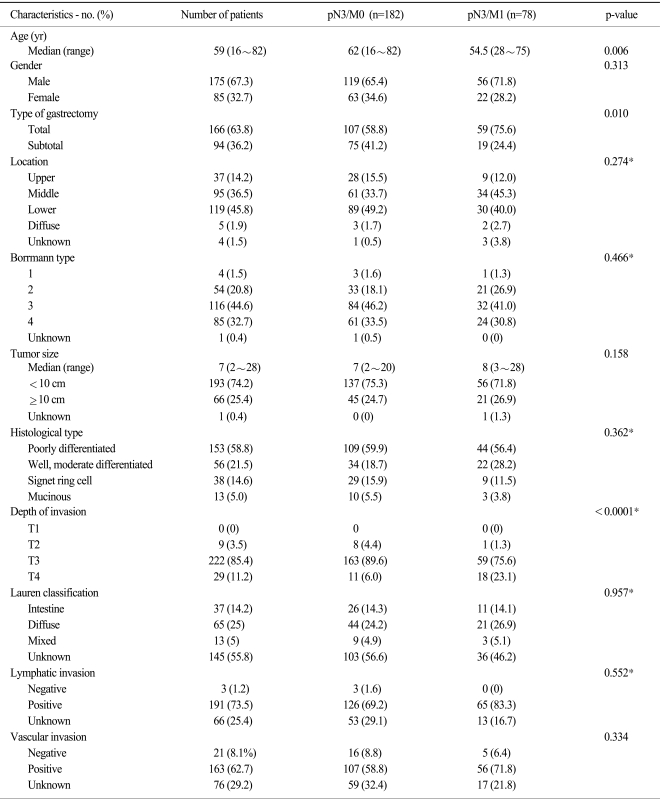
The number of patients who underwent curative resection and who were confirmed as having pN3 stage disease was 182 (70%) and the number of pN3 with M1 stage patients was 78 (30%). Among them, only 12 of the patients were pathologically confirmed as having paraaortic lymph node metastasis (Fig. 1). Thirty-one patients had peritoneal seeding and 18 patients had hepatic metastasis or other organ metastasis.
The most common site of gastric cancer was the lower third of the stomach (45.8%) followed by the middle third of the stomach (36.5%) and the upper third (14.2%). Twenty five percent of the patients had the diffuse type tumor according to Lauren classification. The median tumor size of all the patients was 7 cm (range: 2~28 cm). For the depth of invasion, no cases of T1 stage existed, while the number of T2, T3 and T4 stages was 9 (3.5%), 222 (85.4%) and 29 (11.2%), respectively. Among the clinico-pathologic parameters, there were significant differences between the pN3/M0 patients and the pN3/M1 patients for age (p=0.006), the type of gastrectomy (p=0.010) and the depth of invasion (p<0.0001).
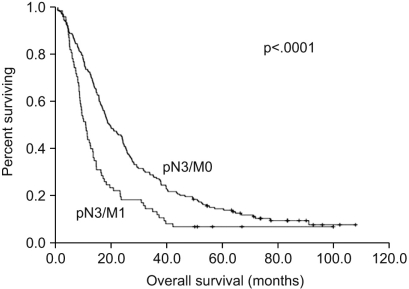
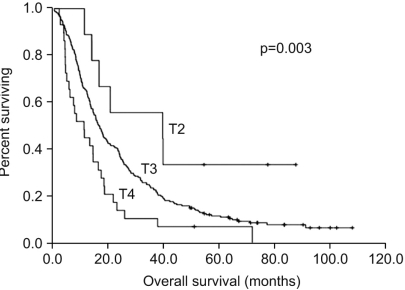
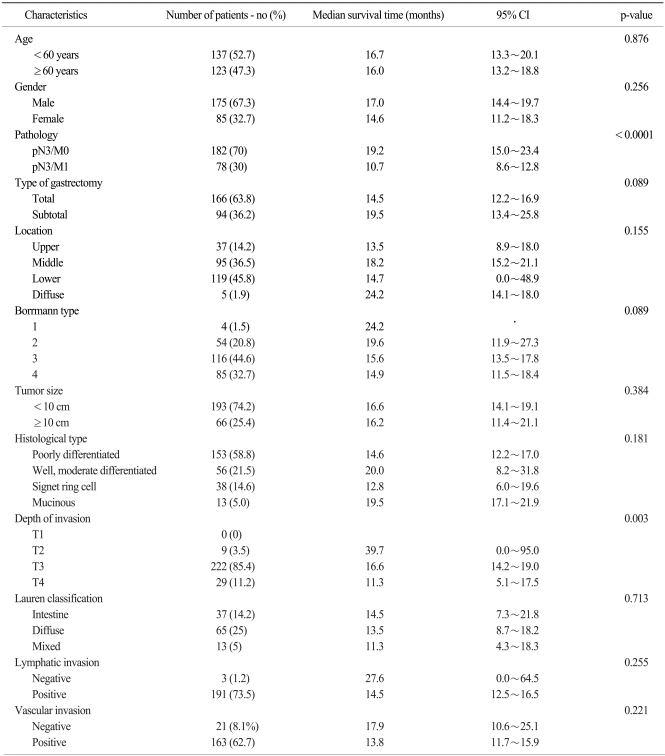
With a median follow-up period of 19 months (range: 1~108), the median overall survival time of all the patients was 16.2 months (95% CI, 14.1~18.3%). The overall 3-year and 5-year survival rates were 23.1% and 9.6%, respectively. When we compare the survival between the two groups, the survival rate of the pN3/M0 group was higher than that of the pN3/M1 group with the 3-year survival rates being 27.5% vs. 12.8%, respectively, and the 5-year survival rates were 12.6% vs. 2.6%, respectively. The median survival time of the pN3/M0 group was 19.2 months (95% CI, 14.9~23.4%) and this was significantly longer as compared with the median survival time of 10.7 months for the pN3/M1 group (95% CI, 8.5~12.8%, p<0.0001) (Fig. 2). For the depth of invasion, an advanced T stage showed a worse prognosis (p=0.003) and the median survival times for T2 stage, T3 stage and T4 stage were 39.7 months (95% CI, 0.00~95.02%), 16.6 months (95% CI, 14.21~18.99%) and 11.3 months (95% CI, 5.15~17.45%), respectively (Fig. 3).
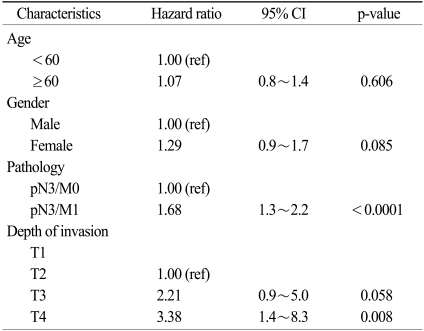
The 3-year and 5-year survival rates and the median survival times according to the clinic-pathologic factors that were analyzed are summarized in Table 2. The results of the multivariate analysis are shown in Table 3.
The factors influencing survival for the patients with pN3 stage disease were the pN3/M1 group (p<0.0001) and the depth of invasion (p=0.003). For all the patients, a multivariate analysis that including four factors (gender, age, the pN3/M1 group and the depth of invasion) from the univariate analysis showed that an advanced T stage was an independent prognostic factor for survival (Table 3). The pN3/M1 group had a higher hazard ratio than the pN3/M0 group (hazard ratio: 1.68, 95% CI, 1.26~2.24, p<0.0001). The hazard ratio for the depth of invasion was 2.21 (95% CI, 0.98~5.03, p=0.058) for the T3 stage and it was 3.38 (95% CI, 1.38~8.31, p=0.008) for the T4 stage. The factor influencing survival for the pN3/M0 patients was the Borrmann type (p=0.039), but the depth of invasion was not a significant factor for survival (p=0.104, Table not shown). There were no significant factors for the pN3/M1 patients. A multivariate analysis for the pN3/M0 patient group included three factors (gender, age and the Bormann type) from the univariate analysis, but no factors were proven to be statistically significant.
The prognosis of stage IV gastric cancer has been thought to be poor and the benefits of surgery are controversial. Some study have reported longer survival for patients with resected stage IV gastric cancer (10), and the selected patients with stage IV gastric cancer with acceptable risk should be considered for surgical resection (11). In addition, Medina-Franco et al. have reported low surgical mortality and low morbidity rates when patients with stage IV gastric cancer undergo surgical resection (6). Radical resection is the standard care for gastric cancer. Some gastric cancer patients undergo gastrectomy with a curative aim because they are diagnosed with a tumor at a resectable stage via imaging studies. Yet some are classified into pathologic stage IV due to their pN3 stage with or without M1 disease, according to the AJCC sixth staging system.
The fifth edition of AJCC cancer staging system for gastric cancer is generally thought to be a good predictor for the prognosis. Compared to the fourth edition, the N3 stage is based on the number of positive metastasis lymph nodes, and TanyN3M0 and T4N1M0 are defined as stage IV in the fifth edition. Stage migration has been detected with a change of the staging system. Klein et al. reported that some of the node-positive patients changed to another N stage, including a higher stage, as compared to the fourth edition (5). In this study, the fifth edition's N staging was suggested to be the most significant prognostic variable. Some studies have also reported the fifth edition of the new pN classification showed more homogenous survival than the old classification (12).
However, some researchers have suggested that stage IV has heterogeneous prognoses for several groups of patients. Lin et al. reported that the patients with the N3 stage and who underwent palliative gastrectomy had much higher 1-year and 2-year survival rates than the patients who underwent operation without resection (bypass or laparotomy exploration only). In this study, the 1 year survival rate of the patients with N3 lymph node metastasis was 66.7%, which was comparable to the 64.4% 1 year survival rate of our study (13). Park el al. suggested sub-classifying stage IV gastric cancer into IVa (T1-3N3M0, T4N1-2M0) and IVb (T4N3M0, TanyNanyM1) for better prediction of survival (7). This study showed that the survival of patients with T1-3N3M0 and T4N1-2M0 stage disease was significantly longer than that of the patients with stage T4N3M0 and stage M1 disease. Li et al divided stage IV gastric cancer into four groups in the same way (14). In addition, An et al. reported on 1056 patients who were divided into three groups: the T4N1M0, T1-3N3M0 and TanyNanyM1 stage groups (15). The patients with the T1-3N3M0 stage showed a better prognosis than the patients with the T4N1-3M0 or the TanyNanyM1 stage. Our analysis also showed a significant difference of survival between the pN3/M0 group and the pN3/M1 group (19.2 months vs. 10.7 months, respectively, p<0.0001).
Many studies have reported various prognostic factors for patients with stage IV gastric cancer. We analyzed the clinic-pathologic features that were correlated with the survival of pN3 gastric cancer patients. We found that the depth of invasion was a significant independent prognostic factor for survival for pN3 gastric cancer patients. As a result, the T4 stage was a poorer prognostic factor than the T2 or T3 stage in the patients with pN3 stage gastric cancer. It is well known that the status of lymph node metastasis is one of the most significant prognostic factors for gastric cancer patients. However, for precisely evaluating the lymph node metastasis status, wide dissection is necessary with a large enough number of dissected lymph nodes. All of the patients in our study underwent D2 or D3 lymphadenectomy, and the numbers of dissected lymph nodes were more than 15 (range: 26 to 165). Hence, in the current study, the lymph node status was not significant for predicting survival, and this suggests the need for developing different parameters for lymph node metastasis for better prediction of survival among the pN3 patients.
Some studies have also reported that Borrmann type IV is an independent prognostic factor (16,17). Our data showed shorter median survival duration for advanced Borrmann type IV, but the differences were not statistically significant. In addition, when we divided the patients based on the tumor size of 10 cm, there was no survival difference between the groups, and this was different from a previous report (18). Although there is no internationally accepted standard of care after cancer surgery, the efficacy of postoperative chemotherapy cannot be ignored as a prognostic factor for pN3 stage patients. There have been some studies that showed that postoperative chemotherapy after D2 surgery was effective (19). In our study, we couldn't find a significant survival difference between the patients who received postoperative chemotherapy and those who didn't because the majority of our patients received postoperative chemotherapy.
We included the sub-classified groups (the pN3/M0 and pN3/M1 groups) as a statistical variable and a significant difference of survival between the pN3/M0 and pN3/M1 groups was seen. We suggest that pN3 stage gastric patients have a different prognosis and we should treat gastric cancer patients with a pN3 stage only (pN3/M0) with aggressive surgical therapy. But, our patients were somewhat limited to the pN3 stage after gastrectomy and our study didn't include palliative resection for other stage IV patients. There are questions about the survival advantage of performing resection for stage IV patients, except for pN3 stage patients.
Although the pN3 stage patients in our study were stage IV, there is a significant difference in survival between the pN3/M0 group and the pN3/M1 group. The survival time is significantly longer for the pN3/M0 group after gastrectomy than for the pN3/M1 group, suggesting a different tumor burden and degree of tumor extension. Therefore, diverse treatment strategies are needed for these heterogeneous pN3 gastric cancer patients to improve their survival.
References
1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006; 24:2137–2150. PMID: 16682732.

2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108. PMID: 15761078.

3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96. PMID: 18287387.

4. Hartgrink HH, Putter H, Klein Kranenbarg E, Bonenkamp JJ, van de Velde CJ. Value of palliative resection in gastric cancer. Br J Surg. 2002; 89:1438–1443. PMID: 12390389.

5. Klein Kranenbarg E, Hermans J, van Krieken JH, van de Velde CJ. Evaluation of the 5th edition of the TNM classification for gastric cancer: improved prognostic value. Br J Cancer. 2001; 84:64–71. PMID: 11139315.

6. Medina-Franco H, Contreras-Saldívar A, Ramos-De La Medina A, Palacios-Sanchez P, Cortés-González R, Uqarte JA. Surgery for stage IV gastric cancer. Am J Surg. 2004; 187:543–546. PMID: 15041508.

7. Park JM, Park SS, Mok YJ, Kim CS. pN3M0 gastric cancer: the category that allows the sub-classification of stage-IV gastric cancer (IVa and IVb). Ann Surg Oncol. 2007; 14:2535–2542. PMID: 17549571.

8. Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009; 71:127–164. PMID: 19230702.

9. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998; 1:10–24. PMID: 11957040.
10. Takeno S, Noguchi T, Kikuchi R, Sato T, Uchida Y, Yokoyama S. Analysis of the survival period in resectable stage IV gastric cancer. Ann Surg Oncol. 2001; 8:215–221. PMID: 11314937.

11. Lim S, Muhs BE, Marcus SG, Newman E, Berman RS, Hiotis SP. Results following resection for stage IV gastric cancer; are better outcomes observed in selected patient subgroups? J Surg Oncol. 2007; 95:118–122. PMID: 17262741.

12. Yoo CH, Noh SH, Kim YI, Min JS. Comparison of prognostic significance of nodal staging between old (4th edition) and new (5th edition) UICC TNM classification for gastric carcinoma. International Union Against Cancer. World J Surg. 1999; 23:492–497. discussion 497-8. PMID: 10085399.
13. Lin SZ, Tong HF, You T, Yu YJ, Wu WJ, Chen C, et al. Palliative gastrectomy and chemotherapy for stage IV gastric cancer. J Cancer Res Clin Oncol. 2008; 134:187–192. PMID: 17611776.

14. Li C, Yan M, Chen J, Xiang M, Zuh ZG, Lin YZ. Prognostic influence of sub-stages according to pTNM categories in patients with stage IV gastric cancer. J Surg Oncol. 2009; 99:324–328. PMID: 19204941.

15. An JY, Ha TK, Noh JH, Sohn TS, Kim S. Proposal to subclassify stage IV gastric cancer into IVA, IVB, and IVM. Arch Surg. 2009; 144:38–45. discussion 45. PMID: 19153323.

16. An JY, Kang TH, Choi MG, Noh JH, Sohn TS, Kim S. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J Gastrointest Surg. 2008; 12:1364–1369. PMID: 18516653.

17. Yook JH, Oh ST, Kim BS. Clinicopathological analysis of borrmann type IV gastric cancer. Cancer Res Treat. 2005; 37:87–91.

18. Kunisaki C, Makino H, Takagawa R, Oshima T, Nagano Y, Kosaka T, et al. Tumor diameter as a prognostic factor in patients with gastric cancer. Ann Surg Oncol. 2008; 15:1959–1967. PMID: 18369676.

19. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820. PMID: 17978289.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download