Abstract
The vast majority of patients with metastatic prostate cancer present with bone metastases and high prostate specific antigen (PSA) level. Rarely, prostate cancer can develop in patients with normal PSA level. Here, we report a patient who presented with a periureteral tumor of unknown primary site that was confirmed as prostate adenocarcinoma after three years with using specific immunohistochemical examination. A 64-year old man was admitted to our hospital with left flank pain associated with masses on the left pelvic cavity with left hydronephrosis. All tumor markers including CEA, CA19-9, and PSA were within the normal range. After an exploratory mass excision and left nephrectomy, the pelvic mass was diagnosed as poorly differentiated carcinoma without specific positive immunohistochemical markers. At that time, we treated him as having a cancer of unknown primary site. After approximately three years later, he revisited the hospital with a complaint of right shoulder pain. A right scapular mass was newly detected with a high serum PSA level (101.7 ng/ml). Tissues from the scapular mass and prostate revealed prostate cancer with positive immunoreactivity for P504S, a new prostate cancer-specific gene. The histological findings were the same as the previous pelvic mass; however, positive staining for PSA was observed only in the prostate mass. This case demonstrates a patient with prostate cancer and negative serological test and tissue staining that turned out to be positive during progression. We suggest the usefulness of newly developed immunohistochemical markers such as P504S to determine the specific primary site of metastatic poorly differentiated adenocarcinoma in men.
Cancer of unknown primary (CUP) is defined by the presence of metastatic disease without an identifiable primary tumor site on presentation, and the incidence of CUP is approximately 2~5% of all cancers (1). Various pathological and imaging procedures have been developed to detect the primary site of CUP, but the detection rate is still low. Only less than 20% of patients have a primary site that has been identified antemortem (1).
A prostate carcinoma is the eighth common cause of cancer death in men in South Korea (2), and almost 40% of patients that present first with prostate cancer have metastatic disease. In cases of metastatic adenocarcinoma, identification of the prostatic origin is important, as metastatic prostate cancer is often responsive to hormone therapy. Although the majority of patients with metastatic prostate cancer present with bone involvement and increased serum prostate specific antigen (PSA) level, a small proportion of patients presents with an unusual pattern of metastasis or normal serum PSA level. We report here a patient who presented with a periureteral tumor of unknown primary site that was confirmed as adenocarcinoma of the prostate three years later with the use of specific immunohistochemical staining.
A 64-year old man was admitted to our hospital with left flank pain on December 2004. On imaging work-up for left flank pain, a computed tomography (CT) scan of the abdomen showed a pelvic mass on the left external iliac area and left hydronephrosis (Fig. 1). A positron emission tomography (PET) image revealed a "hot uptake" mass in the left pelvic cavity (Fig. 1). On laboratory studies, hematological, hepatic, renal function tests and levels of tumor markers including CEA, CA19-9 and PSA were all within the normal range. The patient underwent an exploratory laparotomy and surgical removal of the tumor mass with a left nephrectomy. The histological finding of the removed mass was a poorly differentiated carcinoma. Immunohistochemical staining demonstrated the status of specific markers as pan-cytokeratin (CK) (+), CK 7 (+), CK 20 (-), CEA (±), neuron-specific enolase (NSE) (-), PSA (-) and calretinin (-). According to these immunohistochemical findings, the primary site of the mass was suggested to be from the lung, thyroid, breast or pancreas (3). Pulmonology and gastrointestinal tract examinations were performed to search for the primary lesion. However, the primary site could not be identified. At that time, the patient was diagnosed with cancer of unknown primary and the patient underwent platinum based systemic chemotherapy for six cycles. As seen on follow-up imaging studies including PET, there were no new lesions seen that suggested a recurrent or primary cancer.
Approximately three years later, the patient was readmitted with right shoulder pain. On imaging studies, multiple bone-involved masses were seen on a chest CT scan and bone scan (Fig. 2). From the laboratory findings, the serum PSA was elevated to 101.7 ng/ml. Ultrasound-guided needle biopsies on the right scapular mass and prostate were performed. The histological features of the tissue from the right scapular mass were suggestive of a poorly differentiated carcinoma similar to that of the previously removed pelvic mass. A tissue sample from prostate core needle biopsy was consistent with adenocarcinoma, which showed a raggedly outlined cribriform growth pattern (Gleason score 4) or had infiltrated to the stroma in the form of small clusters without glandular differentiation (Gleason score 5) and showed hyperchromatic nuclei with prominent nucleoli and a monotomous appearance. The prostate tumor cells showed similar histological feature as the new scapular and previous pelvic masses. Immunohistochemical staining for CK7, CK20, PSA and P504S was performed to confirm the same origin of the three masses. Immunoreactivity of tumor cells from the scapular and pelvic masses was identical with CK 7 (+), CK 20 (-), PSA (-) and P504S (+), but the immunoreactivity of tumor cells from the prostate was CK7 (+), CK20 (-), PSA (+) and P504S (+) (Fig. 3).
The patient was finally diagnosed with metastatic prostate cancer three years after the first visit for an unknown primary tumor with positive immunoreactivity for P504S, a new prostate cancer specific gene. Hormonal treatment was started with the administration of luteinizing hormone releasing hormone agonist and anti-androgen. After two months, the serum PSA level was normalized, and followup chest CT showed that masses on the right scapular and anterior chest wall had all decreased significantly in size.
Unknown primary tumor represents a heterogeneous feature of metastatic tumors, and histological presentations of CUP are predominantly classified as adenocarcinomas (50~60%) or poorly differentiated adenocarcinomas or carcinomas (30~40%) (4). The histological types of CUP are a challenge for clinicians to manage as identification of the primary sites is difficult using only routine histological examinations. Recently, advances in pathology using novel immunohistochemical markers can assist the physician with a decision for further diagnostic work-up and clinical care.
Monoclonal antibodies to specific cytokeratin (CK) subtypes have been used in an attempt to classify tumors according to the site of origin. The two most common CK stains used for CUP are CK7 and CK20, and the combination of CK7 and CK20 immunoprofiling has been helpful to identify primary tumor sites (3). For example, a CK7 (-)/CK20 (+) phenotype is often associated with carcinomas of colorectal origin, whereas a CK7 (+)/CK20 (-) phenotype is seen in a wide variety of carcinomas, including carcinomas of the lung, breast, thyroid, pancreas and female genital tract. In the present case, CK stains of the initial pelvic mass demonstrated a marker status of pan-cytokeratin (+), CK7 (+), and CK20 (-). Although the mass was ultimately identified as a prostate adenocarcinoma, at that time, possible origins of the tumor were suggested as the lung, breast, thyroid, or pancreas according to the CK immunoprofiling. However, imaging studies including the use of PET did not show any significant lesions at these suggested organs. Usually, CK profiles of prostate adenocarcinoma are negative for both CK7 and CK20, but no immunohistochemical test is 100% specific, such as in the present case.
The most effective method for the detection of prostate cancer is serum PSA testing with tissue examination of the prostate. However, PSA, which is the most commonly used tumor marker in prostate cancer, is not a cancer specific marker as it is present in benign and malignant prostatic epithelial cells. Serum PSA levels are frequently elevated in benign conditions such as benign prostatic hyperplasia and prostatitis (5). In contrast as in the present case, prostate cancer may develop in patients with normal PSA level (6). Moreover, with a tissue diagnosis as a confirmative method of prostate cancer, it can be difficult to distinguish prostate cancer from other conditions in some cases. As in serologic PSA test, PSA immunohistochemical staining is frequently used to confirm the presence of prostate cancer. The sensitivity and specificity of PSA immunohistochemical stain for prostate cancer is known to be variable according to the histological differentiation of tumor cells (7,8). Goldstein (7) studied the immunophenotypic characterization of prostate adenocarcinoma with intermediate or high Gleason scores (6 to 10), and showed that the PSA immunoreactivity of prostate adenocarcinoma with a Gleason score of 8, 9 or 10 was 58.7% as compared with 99% reactivity of prostate adenocarcinoma with a Gleason score 6 or 7. In that study, CK7 and CK20 immunoreactivity were similar according to the Gleason scores; CK7 and CK20 were reactive in 11% and 20% for Gleason score 9 or 10 patients as compared with 0% and 2.3% for Gleason scores 6, 7, or 8. These results were very similar as with the present case: CK7 (+), CK20 (-), and PSA (-) with Gleason score 9.
In the present case, unusually, PSA immunoreactivity was only observed in the prostate tissue but not in both the metastatic scapular and pelvic masses. These findings are uncommon and sometimes further evaluation was needed to distinguish a double primary cancer. However, the reactivity of PSA staining tends to correlate inversely with the histological differentiation of tumor cells, and different PSA immunoreactivities between primary and metastatic sites have also been reported in a recent study (8). In that study, two distant metastases were found as negative for PSA and two other metastases were weakly positive for PSA among 15 distant metastases, as compared to all PSA positive cases for 20 primary tumors. The investigators suggested that the combined use of PSA and new specific markers for prostate cancer could increase the diagnostic accuracy.
We used a novel marker, P504S to confirm the final diagnosis of the tissues from the primary and two metastatic sites although histological similarity of the three tissues was observed after haematoxylin and eosin staining. P504S/α-methylacyl coenzyme A racemase (AMACR) is one of newly used markers for the diagnosis of prostate cancer and is a successful example of translating an advanced molecular finding into clinical practice. It is the first gene identified by a combination of cDNA subtraction and high throughput microarrary screening from benign and malignant prostate tissue (9). As described in several reports where an anti- P504S/AMACR antibody was utilized, P504S/AMACR has been demonstrated as an important positive tissue marker for prostate cancer regardless of tumor grade, with a sensitivity ranging from 82% to 100% and a specificity ranging from 79% to 100% (10-12).
In cases with a poorly differentiated prostate cancer, these cases are often confused with poorly differentiated urothelial carcinomas. In recent studies, concomitant use of positive markers such as PSA and P504S as well as negative markers such as high molecular weight cytokeratin (HMWCK, 34βE12) and p63 can be helpful in the accurate diagnosis of prostate cancer, in addition to the histological or clinical findings (13,14).
In the present case, although we could not employ negative markers such as HNWCK or p63, the pelvic and scapular masses were finally diagnosed as metastatic prostate cancer according to the histological and immunohistochemical findings. In addition, a good clinical response to hormone therapy supported our diagnosis.
Prostate cancer is often included in the list of possible primary sites of metastatic and poorly differentiated adenocarcinomas, and is a malignancy that shows a good response to hormonal therapy as in the present case. For a more accurate diagnosis, we suggest that the use of newly developed molecular marker, such as P504S, should be considered to confirm the primary site in cases of metastatic poorly differentiated adenocarcinoma that occur in men.
References
1. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003; 39:1990–2005. PMID: 12957453.

2. Ministry of health and welfare. Central cancer registry center. regional cancer registry. Annual report of the cause of death in Korea 2006.
3. Varadhachary GR, Abbruzzese JL, Lenzi R. Diagnostic strategies for unknown primary cancer. Cancer. 2004; 100:1776–1785. PMID: 15112256.

4. Greco FA, Hainsworth JD. DeVita VT, Hellman S, Rosenberg SA, editors. Cancer of unknown primary site. Cancer: principles and practice of oncology. 2005. 7th ed. Philadelphia, PA: Lippincott;p. 2213–2236.
5. Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery: what we have learned and where we are going. J Urol. 1999; 162:293–306. PMID: 10411025.
6. Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004; 350:2239–2246. PMID: 15163773.
7. Goldstein NS. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am J Clin Pathol. 2002; 117:471–477. PMID: 11888088.

8. Sheridan T, Herawi M, Epstein JI, Illei PB. The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. Am J Surg Pathol. 2007; 31:1351–1355. PMID: 17721190.

9. Xu J, Stolk JA, Zhang X, Silva SJ, Houghton RL, Matsumura M, et al. Identification of differentially expressed genes in human prostate cancer using subtraction and microarray. Cancer Res. 2000; 60:1677–1682. PMID: 10749139.
10. Jiang Z, Woda BA, Rock KL, Xu Y, Savas L, Khan A, et al. P504S: a new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol. 2001; 25:1397–1404. PMID: 11684956.
11. Rubin MA, Zhou M, Dhanasekaran SM, Varambally S, Barrette TR, Sanda MG, et al. Methylacyl Coenzyme A Racemase as a tissue biomarker for prostate cancer. JAMA. 2002; 287:1662–1670. PMID: 11926890.

12. Beach R, Gown AM, De Peralta-Venturina MN, Folpe AL, Yaziji H, Salles PG, et al. P504S immunohistochemical detection in 405 prostatic specimens including 376 18-gauge needle biopsies. Am J Surg Pathol. 2002; 26:1588–1596. PMID: 12459625.

13. Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. Am J Surg Pathol. 2007; 31:1246–1255. PMID: 17667550.

14. Martínez-Rodríguez M, Ramos D, Soriano P, Subramaniam M, Navarro S, Llombart-Bosch A. Poorly differentiated adenocarcinomas of prostate versus high-grade urothelial carcinoma of the bladder: a diagnostic dilemma with immunohistochemical evaluation of 2 cases. Int J Surg Pathol. 2007; 15:213–218. PMID: 17478786.

Fig. 1
Abdomen CT and PET scan findings at 1st visit. Abdomen CT (A) showed an approximately 3.0×2.5cm sized conglomerated lymph nodes enlargement (arrow) in left external iliac area with compressing the left distal ureter. PET scan (B) revealed a hypermetabolic mass (SUV=5.3) on the left external iliac node area (arrow).
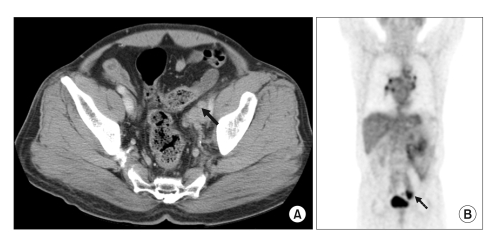
Fig. 2
Chest CT (A, B) and bone scan (C) findings at 3 years later. Chest CT showed a 8.4cm sized mass (arrow) on the right scapular and glenoid fossa (A) and a 2.3cm sized mass (arrow) on the anterior chest wall (B). Bone scan revealed hot uptakes on the ribs and the right scapular (C).
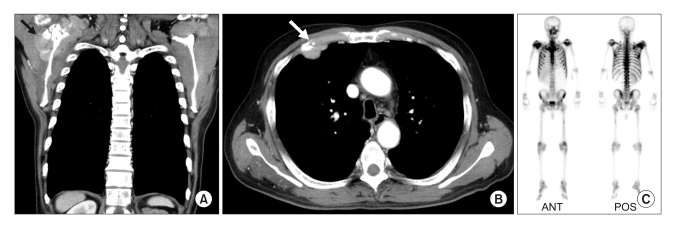
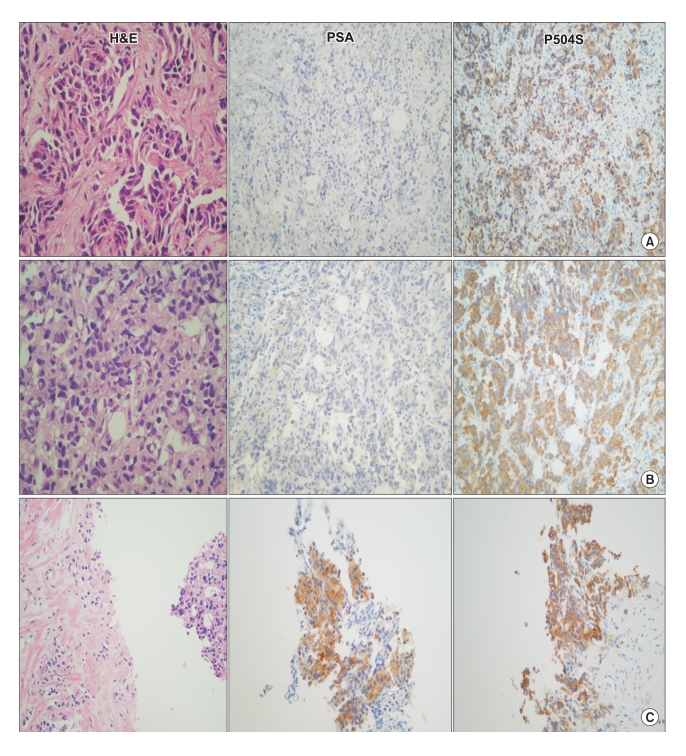
Fig. 3
Pathologic features with immunoistochemical stainings for PSA and P504S (A: pelvic mass, B: scapular mass, C: prostate biopsy). A; The pelvic mass showed poorly differentiated carcinoma cells with hyperchromatic nuclei and small amount of cytoplasm. These tumor cells showed negative immunoreactivity for PSA but positive immunoreactivity for P504S. B; The scapular mass showed infiltrating poorly differentiated carcinoma cells, with the same histologic features and immunoreactivity for PSA and P504S to these of the previous pelvic mass. (A) C; The prostate biopsy demonstrated adenocarcinoma cells, with Gleason score 9 (4+5). Tumor cells demonstrated the same histological features to these of the previous pelvic and scapular masses but positive immunoreactivity for both PSA and P504S.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download